Figure 1. Homology modelling of ShK analogues in complex with Kv1.3.
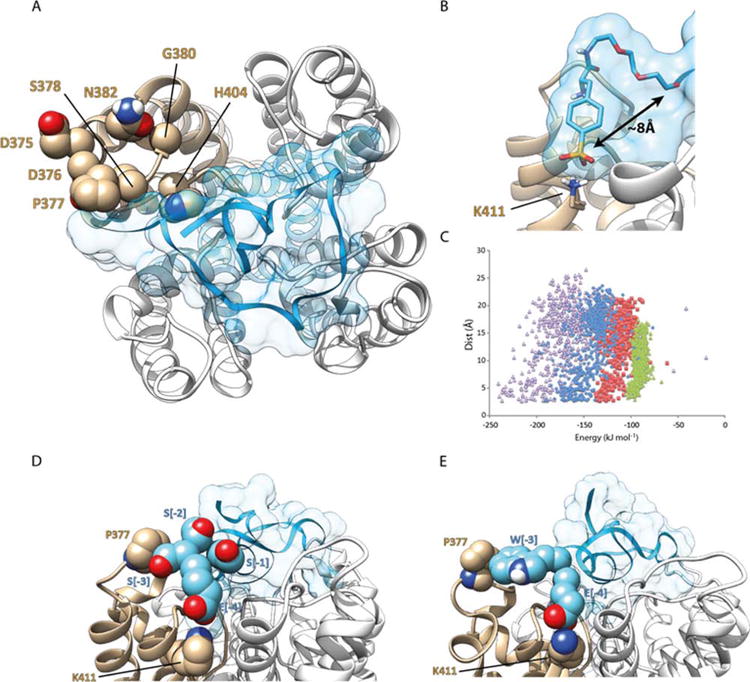
(A) ShK-192 in complex with Kv1.3; view perpendicular to the membrane plane with the channel represented as a white and tan ribbon, and the ShK analogue in blue with a transparent surface. The side chain atoms of residues on the surface of the channel that differ between Kv1.1 and Kv1.3 are illustrated as spheres (atom coloring with carbon tan). (B) The phosphono group of the N-terminal Ppa extension in ShK-192 lies ∼8 Å from the N-terminus of the native ShK and forms a salt bridge with the ammonium of Lys 411. (C) Comparison of MODELLER energies and separation between the N-terminal Glu of the ShK analogue and Lys411 of the channel. Extensions to ShK of 1 (green triangle, E), 2 (red square, ES), 3 (blue diamond, ESS) and 4 (purple triangle, ESSS) residues. (D) Homology model of [ESSS]ShK in complex with Kv1.3. The side-chain atoms of Pro377 and Lys411 of the channel are represented as spheres (atom coloring with carbon tan). The side-chain atoms of the 4-residue extension, ESSS, are represented as spheres (atom coloring with carbon cyan). (E) Homology model of [EWSS]ShK in complex with Kv1.3. The side-chain atoms of Pro377 and Lys411 of the channel are highlighted. The side-chain atoms of the first two residues (EW) of the 4-residue extension are represented as spheres (atom coloring with carbon cyan).
