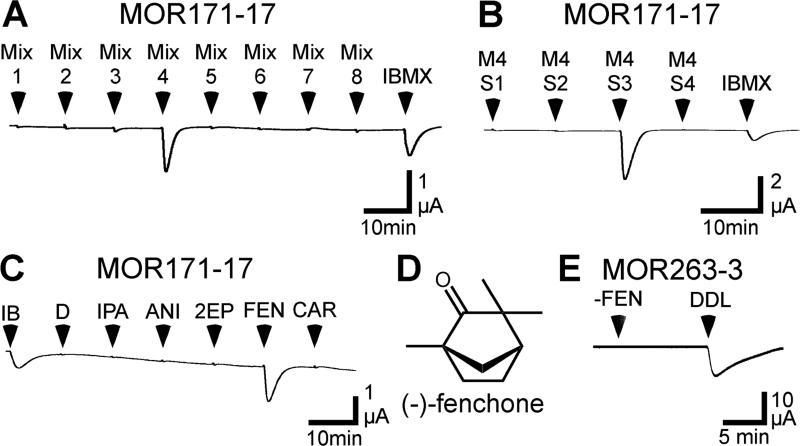
Figure 4. MOR171-17 responds to (−)-fenchone.
A) An oocyte expressing MOR171-17, Gαolf and CFTR was screened with 15 sec applications of 1mM IBMX and 8 mixtures containing a total of 155 odorants. Each mixture contained 17 - 20 odorants, each at a concentration of 30 μM (the odorant composition of each mixture is listed in the Supplemental Table 3). B) An oocyte expressing a MOR171-17, Gαolf and CFTR was challenged with 15 sec applications of 4 sub-mixtures derived from the mixture 4 and 1mM IBMX. M4S1 contained 3-heptanol, (+)-isopulegol, 3-phenyl-1-propanol, trans,trans-2,4-octadienal and cuminaldehyde. M4S2 contained trans,trans-2,4-heptadienal, phenylacetic acid, allyl tiglate, γ-octalactone and heptyl butyrate. M4S3 contained isobutyl phenylacetate, anisole, 2-ethylpyrazine, (−)-fenchone and (+)-carvone. M4S4 contained 3-nonanone, 2,3-pentanedione, 2-isobutylthiazole, 2-thiophenethiol and (−)-β-pinene. Each odorant was present at a concentration of 30 μM. C) An oocyte expressing a MOR171-17, Gαolf and CFTR was challenged with 15 sec applications of 1mM IBMX, 0.006% DMSO and 30 μM of each of the individual odorants from the M4S3: isobutyl phenylacetate (IPA), anisole (ANI), 2-ethylpyrazine (2EP), (−)-fenchone (FEN) and (+)-carvone (CAR). D) Structure of (−)-fenchone. E) An oocyte expressing a MOR263-3, Gαolf and CFTR was challenged with 15 sec applications of 30 μM (−)-fenchone and 30 μM trans,trans-2,4-decadienal.