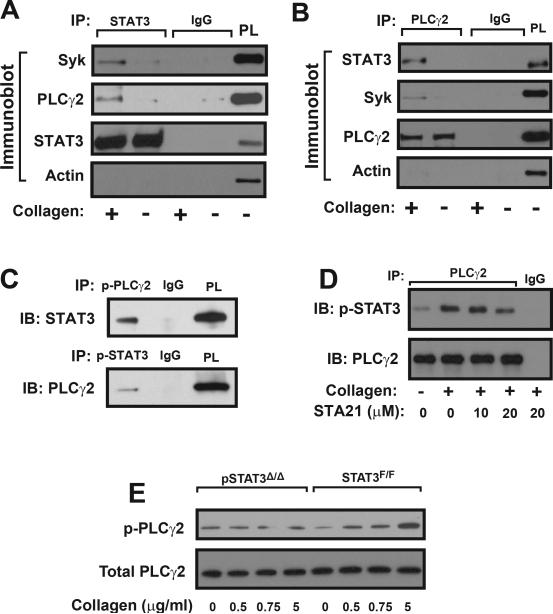
Figure 5.
Co-immunoprecipitation: Washed human platelets were stimulated with 5 μg/ml of collagen and platelet lysates were incubated with a STAT3 antibody followed by immunoprecipitation (IP) with protein A sepharose beads. Precipitated proteins were probed with antibodies to STAT3, Syk, PLCγ2, and actin (A, isotype IgG as control and STAT3 as loading control, platelet lysate [PL] as positive control). The same technique was used to immunoprecipitate PLCγ2 and probe for PLCγ2, Syk, STAT3, and actin (B). STAT3 and PLCγ2 were also immunoprecipitated with antibodies specifically against phosphorylated PLCγ2 and phosphorylated STAT3, respectively (C). STAT3 was co-immunoprecipitated with a PLCγ2 antibody from lysates of platelets stimulated with 5 μg/ml of collagen in the presence of increasing doses of STA21 (D). PLCγ2 phosphorylation was measured in platelets from pSTAT3Δ/Δ and STAT3F/F mice stimulated with increasing doses of collagen (E). Panels in the figure represent of 3–6 separate experiments.