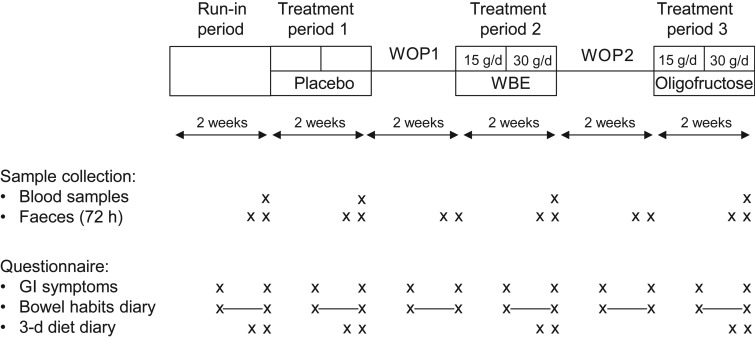
Fig. 1.
Schematic representation of the study design. The study started with a 2-week run-in period, followed by three 2-week treatment periods in which the following study products (not necessarily in the described order) were taken by the volunteers: (i) wheat bran extract (WBE) at a dose of 15 g/d (first week of WBE treatment period) and 30 g/d (second week of WBE treatment period); (ii) oligofructose at a dose of 15 g/d (first week of oligofructose treatment period) and 30 g/d (second week of oligofructose treatment period); and (iii) placebo. The treatment periods were separated by 2-week washout periods (WOP). Blood and faecal samples were collected at the indicated time points. The subjects completed weekly a questionnaire assessing the occurrence frequency and distress severity of eighteen gastrointestinal (GI) symptoms. Additionally, subjects recorded in the bowel habits diary the number of bowel movements and stool consistency during the second week of the run-in period, and each of the treatment periods and washout periods.