Abstract
Independent studies on major depressive disorder (MDD) and hypertension, suggest overlapping abnormalities in brain regions associated with emotional and autonomic processing. However, the unique and interactive effects of MDD and hypertension have never been studied in a single sample. Brain volume in these areas may be an explanatory link in the comorbidity between MDD and hypertension. Voxel-based morphometry was used to test for main effects of MDD (N = 152) and hypertension (N = 82) and their interactions on gray and white matter volumes. Voxel-wise results are reported at p < .05 FWE corrected for the spatial extent of the whole brain and a-priori regions of interest (ROIs: hippocampus, anterior cingulate cortex (ACC) and inferior frontal gyrus (IFG)). In addition, analyses on the extracted total volumes of our ROIs were performed. Interactive effects in the mid-cingulate cortex (MCC) (pFWE = .01), cerebellum (pFWE = .01) and in the ACC total ROI volume (p = .02) were found. MDD in the presence, but not in the absence of hypertension was associated with lower volumes in the ACC and MCC, and with a trend towards larger gray matter volume in the cerebellum. No associations with white matter volumes were observed. Results suggest that the combination of MDD and hypertension has a unique effect on brain volumes in areas implicated in the regulation of emotional and autonomic functions. Brain volume in these regulatory areas may be an explanatory link in the comorbidity between hypertension and MDD.
Keywords: Depression, Blood pressure, Hypertension, Comorbidity, MRI, Gray matter
Highlights
-
•
We tested for main and interaction effects of MDD and hypertension on brain volumes.
-
•
Voxel based morphometry was used to test effects on gray and white matter volumes.
-
•
Both whole brain and region of interest analyses were performed.
-
•
The combination of MDD and hypertension has unique effects on regional brain volumes.
-
•
Brain volume may be a link in the comorbidity between hypertension and MDD.
1. Introduction
The incidence of hypertension is increased in depressed patients compared to the general population (Meng et al., 2012). Moreover, both hypertension and depression increase the risk of incident cardiovascular disease (CVD) and accelerate the progression of CVD (Nemeroff and Goldschmidt-Clermont, 2012; Thayer and Lane, 2007). Multiple biological factors have been proposed to play a role in the association between depression and vascular related diseases (de Jonge et al., 2010), including inflammation, hypothalamus–pituitary–adrenal (HPA) axis dysregulation, and autonomic dysfunction, reflected by decreased heart rate variability. Although both depression and hypertension have independently been associated with abnormalities in brain structure and function, regional brain morphology as a shared biological link has rarely been studied.
Depression has been associated with gray matter (GM) volume loss predominantly in prefrontal-limbic networks, such as the anterior cingulate cortex (ACC), hippocampus, and inferior frontal gyrus (IFG) (Arnone et al., 2012; Bora et al., 2012; Du et al., 2012; Lai, 2013), which have been implicated in processing of emotional information (Groenewold et al., 2013). It is not fully clear whether alterations in regional brain volumes are a cause or consequence of depression, or both. Conceivably, pre-existing abnormalities in brain regions involved in emotion processing may render individuals vulnerable to depression. Alternatively, excessive stress during depressive episodes could have damaging effects on the brain. The association between white matter (WM) volumes and depression has been studied less extensively and has resulted in inconsistent findings (Abe et al., 2010; Kim et al., 2008; Steingard et al., 2002).
Like depression, hypertension is also an established risk factor for brain abnormalities such as WM lesions and decreased GM volumes in prefrontal-limbic areas (Beauchet et al., 2013; Gianaros et al., 2006; Jennings et al., 2012; Jennings and Zanstra, 2009; Maillard et al., 2012; Raz et al., 2003). In addition to the damaging effects of hypertension on the brain, it has been proposed that alterations in regional brain volume may predispose individuals to develop hypertension and cardiovascular disease, due to reduced regulatory control on blood pressure and heart rate during stressful situations (Jennings and Zanstra, 2009). According to this theory, subclinical hypertension would be associated with similar alterations in brain structure as found in clinical hypertension and CVD. Of interest, GM abnormalities in persons with hypertension are reported in structures that correspond to the ‘emotional’ areas implicated in depression, i.e. in prefrontal-limbic areas such as the prefrontal cortex, the ACC, and the hippocampus (Beauchet et al., 2013; Gianaros et al., 2006; Gilbert et al., 2010; Jennings and Zanstra, 2009; Maillard et al., 2012; Raz et al., 2003; Woo et al., 2009). In addition to emotion processing, these regions have also been found to play a role in regulating autonomic functions, such as heart rate and blood pressure (Critchley et al., 2011; Gasquoine, 2013; Gianaros et al., 2008; Parvizi et al., 2013; Williamson et al., 2006).
There may be several explanations for this overlap in brain abnormalities, such as a shared vulnerability for depression and hypertension, in which the same brain regions could be involved in multiple (i.e. both emotional and cardiovascular) processes. In addition, stress is a shared risk factor for the development of high blood pressure, CVD, and depression, and could also potentially impact brain volumes (Baune et al., 2012; Brotman et al., 2007; Grippo and Johnson, 2002; Harrison et al., 2013). Furthermore, it has been suggested that depression in the absence and presence of cardiovascular and metabolic conditions has distinctive subtypes, in terms of genetic predisposition (Kendler et al., 2009), time of onset (Alexopoulos et al., 1997), and symptomatology (Ormel and de Jonge, 2011). Possibly, these subtypes may be associated with distinctive brain morphology. Alternatively, the vascular depression hypothesis posits that brain structural abnormalities, such as WM lesions, as a result of vascular pathology may give rise to depressive symptoms (Alexopoulos et al., 1997; Krishnan et al., 2004; Thomas et al., 2002).
Despite the suggested overlap of brain abnormalities in depression and hypertension, volumetric brain differences associated with hypertension and depression have never been studied simultaneously. The current study investigated whether hypertension and depression share regional volumetric alterations in a whole brain voxel-wise comparison, and in specific regions of interest (ROIs): the hippocampus, inferior frontal gyrus (IFG) and anterior cingulate cortex (ACC). The comorbidity of MDD and hypertension has never been taken into account in previous MRI research. As comorbid depression is associated with accelerated CVD progression (Nemeroff and Goldschmidt-Clermont, 2012), comorbid depression and hypertension may have more advanced vascular pathology, potentially leading to more pronounced volumetric brain alterations. Alternatively, comorbid depression and hypertension may be a vulnerable subgroup characterized by distinctive brain morphology. Therefore, the effects of hypertension and MDD may not be independent, but rather interactive.
2. Materials and methods
2.1. Participants
For this study we used data from the Netherlands Study of Depression and Anxiety (NESDA), a multicenter longitudinal cohort study. This study was approved by the ethical review board of each participating center and all of the participants signed informed consent before inclusion. The design has been described in detail elsewhere (Penninx et al., 2008). Out of 2981 NESDA respondents, 301 native Dutch-speaking participants aged between 18–57 years were asked to participate in the NESDA neuroimaging study if they met the following inclusion criteria: the DSM-IV criteria for a diagnosis of MDD and/or anxiety disorder (panic disorder, social anxiety disorder, generalized anxiety disorder) in the past 6 months, or no life-time DSM-IV diagnosis (except for life-time alcohol and/or drug dependency or abuse) (control group).
The exclusion criteria were: 1) the presence of axis-I lifetime disorders other than MDD or anxiety disorder; 2) use of psychotropic medication other than SSRIs or infrequent benzodiazepine use (i.e. equivalent to 2 × 10 mg oxazepam; 3 times a week, or use within 48 h prior to scanning); 3) presence of major internal and/or neurological disorders (e.g. type 1 diabetes, CVA/TIA); 4) systolic blood pressure >180 mm Hg and/or diastolic blood pressure > 120 mm Hg (because this can affect the brain's hemodynamics and thereby potentially confound functional MRI measurements, which was also part of the NESDA neuroimaging scan-protocol); 5) dependency or recent abuse (past year) of alcohol and/or drugs; 6) use of beta-blockers that may affect the brain's hemodynamics (Carvedilol, Oxprenolol, Pindolol, Bisoprolol, and Nebivolol); 7) general MRI contra-indications.
In total, 301 participants underwent MRI in one of three participating centers, i.e. Leiden University Medical Center (LUMC), Academic Medical Center Amsterdam (AMC) and University Medical Center Groningen (UMCG). Data from patients with CVD (self-reported diagnosis of coronary disease, cardiac arrhythmia, angina, heart failure, or myocardial infarction) was excluded (N = 6), because in this study we specifically examined hypertension. In addition, data from 10 participants was excluded because of poor image quality. Therefore the final sample consisted of 285 participants.
2.2. Hypertension
To assess hypertension, systolic and diastolic blood pressure was measured during supine rest on the right arm, using an OMRON M4 IntelliSense digital blood pressure monitor (HEM-752A, Omron Healthcare, Inc., Bannockburn, Illinois, USA). The participants were classified as hypertensive when one of the following conditions was met: 1) they reported to have hypertension or to receive medication for hypertension, or 2) when the average of two consecutive baseline measures (at intervals of at least 1 min) for blood pressure exceeded a systolic value of 140 mm Hg, or 3) when blood pressure exceeded a diastolic value of 90 mm Hg, which are commonly used thresholds for hypertension (Pickering et al., 2005). For interpretation purposes, we used hypertension as a categorical measure, as descriptive variables and brain volumes can then be compared across groups. Moreover, we were specifically interested in a clinically relevant measure, that also included currently normotensive participants with treated hypertension. Furthermore, we chose to incorporate systolic and diastolic blood pressure in one measure, as these were highly correlated in the current sample (r = .75).
2.3. Depressive disorder
All participants were interviewed with the Composite International Diagnostic Interview (CIDI) version 2.1 to establish the presence of depressive and anxiety disorders according to the Diagnostic and Statistical manual of Mental disorders fourth edition (DSM-IV) (Kessler and Ustun, 2004), administered by trained interviewers. In the current study analyses were focused on Major Depressive Disorder (half-year recency), while anxiety (Social Anxiety Disorder, Panic Disorder with or without Agoraphobia, and/or Generalized Anxiety Disorder; half year recency) was accounted for by including it as a covariate.
2.4. Other variables
Information about baseline characteristics was obtained from interviews, clinical measurements, and questionnaires. Age, sex, years of education, presence of type 2 diabetes, and medication use were determined during an interview. Measures of weight and length were used to calculate body mass index and Doppler measures of blood pressure of the arm and ankle were used to calculate ankle-brachial index (ABI), a measure of peripheral arterial disease. Metabolic syndrome was defined according to the revised guidelines of the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2001; Grundy et al., 2005), described in detail previously (Klabbers et al., 2010). In short, metabolic syndrome was defined when 3 of the following abnormalities were present: increased waist circumference, (treatment for) increased triglycerides, (treatment for) reduced high-density cholesterol, increased blood pressure or treatment for hypertension, and (treatment for) elevated glucose levels. A questionnaire was used to verify smoking status. The severity of depression was assessed by the Inventory of Depressive Symptoms (IDS). In addition, the groups with MDD might be characterized by different depressive symptom profiles. Therefore, the IDS was divided into two depressive symptom dimensions: a mood–cognition subscale and an anxiety–arousal subscale, which were identified previously in this sample by principal component analysis and confirmatory factor analysis (Wardenaar et al., 2010).
2.5. MRI data acquisition
Participants were scanned in Philips 3-Tesla MRI scanners located at each of the three participating centers, equipped with either a SENSE-6 or a SENSE-8 channel head coil. Anatomical images were acquired by using a 3-dimensional gradient-echo T1-weighted sequence with the following parameters: 170 slices; repetition time = 9 ms; echo time = 3.5 ms; matrix: 256 × 256; voxel size: 1 × 1 × 1 mm; scan duration = 4.5 min.
2.6. Data preprocessing
Imaging data were analyzed with Statistical Parametric Mapping software (SPM 12), implemented in Matlab 7.8.0. The images were manually reoriented to the anterior commissure and segmented into GM, WM, cerebrospinal fluid, skull, and soft tissue outside the brain, using the standard segmentation option in SPM 12, including the ‘light clean-up’ setting. Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) was used for normalization and modulation of the images. DARTEL is a recommended method to increase the accuracy of inter-subject alignment by modeling the shape of each brain (Ashburner, 2007). First, a DARTEL template was created based on the deformation fields that are produced during the segmentation procedure. Next, all individual deformation fields were registered to this template. After this, the obtained deformed images were used to generate smoothed, spatially normalized, and Jacobian scaled gray and white images in MNI space. To increase the signal to noise ratio, the GM and WM images were smoothed using an 8 mm full-width-half-maximum Gaussian kernel.
2.7. Statistical analyses
Voxel-based morphometry (VBM) analyses were masked to exclusively select true positive gray and white matter voxels and to optimize sensitivity, by using the Masking toolbox (Ridgway et al., 2009). In this study a 2 × 2 ANOVA analysis was performed to test for the main and interaction effects of hypertension and MDD. Scan location, age, sex, and the current presence of anxiety disorder were entered as covariates in each comparison, as these might confound the findings. Furthermore, we examined whether adding selective serotonin/norepinephrine re-uptake inhibitor (SSRI/SNRI) use as a covariate would change the results, as SSRIs and SNRIs might affect brain volumes.
For effects occurring in our a-priori regions of interest (ROIs, i.e. the anterior cingulate cortex (ACC), the inferior frontal gyrus (IFG) and the hippocampus); based on previous meta-analyses on volumetric differences in MDD (Arnone et al., 2012; Bora et al., 2012; Du et al., 2012; Lai, 2013), a composite mask was created using the WFU PickAtlas and encompassed these regions as defined by the corresponding automated anatomical labeling (AAL) system labels. Volumetric studies in the hypertension literature often analyzed total ROI volumes instead of employing voxel-based analyses (Beauchet et al., 2013; Jennings et al., 2012; Woo et al., 2009), which is more common for the depression literature. To be able to compare our results to both fields we analyzed our ROIs as total volumes as well as voxel-based. For the total ROI-based analyses, we extracted GM volumes of the individual bilateral ROIs (i.e. full spatial extent of the AAL labels) and exported these ROI volumes to SPSS 20.0 to perform ANCOVA. The VBM ROI analyses were performed in SPM, in which multiple comparison correction was restricted to the spatial extent of composite mask of the ROIs. Effects occurring outside these ROIs had to meet whole-brain correction for multiple comparisons. All VBM results are reported at p <0.05 FWE cluster-corrected, with the initial voxel threshold at p < 0.005 and a spatial cluster extent threshold of k > 50. Non-stationarity correction was applied to correct for non-uniformity in image smoothness (http://fmri.wfubmc.edu/cms/software#NS) in all analyses.
VBM analyses were corrected for total gray and white matter volumes (i.e. Total Brain Volume (TBV)), by using the global values to proportionally scale the original voxel values. In the SPSS ROI analyses, TBV was included as a covariate. SPSS was also used to analyze the demographic and clinical data, using ANOVA and Chi-square with alpha set at p < 0.05.
3. Results
3.1. Baseline characteristics
The total sample consisted of 285 participants (mean age: 37; SD: 10). The baseline characteristics are listed in Table 1 for 4 groups: 1) no hypertension and no MDD (N = 92); 2) no hypertension and MDD (N = 111); 3) hypertension and no MDD (N = 41); 4) both hypertension and MDD (N = 41) (Table 1). Participants with hypertension were significantly older and less likely to be female, and had a higher BMI than participants without hypertension. Participants with MDD had less years of education and were more likely to use SSRIs and SNRIs. Furthermore, depressive participants with comorbid hypertension had a later age at onset of depression and a more severe depression, but did not differ on symptom dimensions compared to depressive participants without hypertension. Hypertensive participants with comorbid depression had a lower average systolic blood pressure compared to hypertensive participants without depression.
Table 1.
Baseline characteristics.
| HT − MDD − N = 92 |
HT − MDD + N = 111 |
HT + MDD − N = 41 |
HT + MDD + N = 41 |
p | |
|---|---|---|---|---|---|
| Age (SD) | 36.5 (9.3) | 35.0 (10.1) | 41.1 (9.8) | 42.6 (9.5) | <.001 |
| Female (%) | 74 (80%) | 82 (74%) | 18 (44%) | 19 (46%) | <.001 |
| Education years (SD) | 13.7 (2.9) | 12.3 (3.0) | 13.2 (3.5) | 12.1 (3.1) | .003 |
| Scan location (%AMC/LUMC/UMCG) | 28/41/30 | 28/41/31 | 34/39/27 | 32/32/37 | .92 |
| Handedness (% left-handed) | 7 (7.6%) | 10 (9.0%) | 3 (7.3%) | 2 (4.9%) | .87 |
| SSRI/SNRI (%) | 12 (13%) | 39 (35%) | 9 (22%) | 17 (42%) | .001 |
| Current anxiety disorder (%) | 46 (50%) | 64 (58%) | 20 (49%) | 23 (56%) | .64 |
| Systolic blood pressure (SD) | 124 (9) | 123 (10) | 152 (12) | 146 (13) | <.001 |
| Diastolic blood pressure (SD) | 76 (7) | 75 (8) | 90 (10) | 88 (8) | <.001 |
| Self-reported treatment for hypertension (%) | – | – | 12 (29%) | 10 (24%) | .62 |
| Age at onset of depression | – | 22 (10) | – | 29 (12) | <.001 |
| Age at onset of hypertension | – | – | 38 (10) | 38 (7) | .95 |
| Diabetes (%) | 0 (0.0%) | 3 (2.7%) | 1 (2.4%) | 3 (7.3%) | .09 |
| Body mass index (SD) | 24.0 (4.1) | 24.8 (4.2) | 25.7 (5.1) | 27.4 (5.1) | .001 |
| Metabolic syndrome (%) | 3 (3.3%) | 6 (5.5%) | 7 (17.1%) | 13 (31.7%) | <.001 |
| Ankle-brachial index ≤0.9 (%) | 1 (1.1%) | 4 (3.6%) | 2 (5%) | 1 (2.5%) | .60 |
| Current smoker (%) | 23 (25%) | 39 (35%) | 16 (39%) | 17 (42%) | .18 |
| Depression severity IDS score (SD) | 13.1 (11.7) | 29.7 (10.6) | 15.5 (13.2) | 34.5 (11.3) | <.001 |
| Cognitive–mood factor (SD) | 3.8 (4.0) | 9.6 (3.1) | 4.7 (4.5) | 10.3 (3.1) | <.001 |
| Anxiety–arousal factor (SD) | 3.3 (2.6) | 6.1 (2.0) | 3.5 (3.0) | 6.4 (2.0) | <.001 |
HT = hypertension; MDD = major depressive disorder; SD = standard deviation; AMC = Amsterdam Medical Center; LUMC = Leiden University Medical Center UMCG = University Medical Center Groningen; SSRI/SNRI = selective serotonin/norepinephrine re-uptake inhibitors (SSRI/SNRI); IDS =inventory of depressive symptomatology. p-Values are based on F-test and chi square.
3.2. ROI-based analyses
We did not observe main effects for hypertension in the SPSS total ROI volume analyses, in which mean volumes of each of the total ROIs were analyzed. There was a main effect for MDD, in which persons with MDD had lower total GM in the ACC (p = 0.01). In addition, there was an interaction effect in this region (p = 0.02) (Table 2a and Fig. 1). In order to be able to interpret the effect of MDD in the context of an interaction with hypertension, the effect of MDD was stratified on hypertension; MDD with hypertension was significantly associated with lower GM volume in the ACC (p = 0.01), but not MDD without hypertension (p = 0.83). In Fig. 1 it can be observed that the comorbid group had the lowest ACC volume of all groups. There were no significant findings for the hippocampus and IFG total volumes.
Table 2A.
SPSS ANOVA results of total ROI volumes for GM; regions of interest tested: bilateral anterior cingulate cortex, inferior frontal gyrus, and hippocampus.
| Anterior cingulate cortex | F | p |
|---|---|---|
| HT− = HT+ | .008 | .930 |
| MDD− > MDD+ | 6.3 | .012 |
| HT ∗ MDD | 5.5 | .020 |
HT = hypertension; MDD = major depressive disorder.
Comparisons in the analyses: the main effects of HT and MDD and interaction effects of HT ∗ MDD were tested in 1 model and adjusted for scan location, age, sex, anxiety disorder, and whole brain volume (df: 1, 276).
Fig. 1.

Location ROI anterior cingulate cortex and total GM volumes per group (in ml). Post-hoc stratification analyses on hypertension showed that only MDD with hypertension appeared to be significantly associated with reduced GM volume in the ACC (F = 7.1; p = 0.01), but not MDD without hypertension (p = 0.83).
3.3. VBM results
In contrast to the ROI-based analyses in SPSS, there were no significant effects in any of the ROIs in the VBM analyses after family-wise error correction. There was a marginal interaction effect in the ACC (Table 2b), in which a similar pattern was observed as for the ACC in the ROI based analyses (figures not shown). To a lesser extent, this was also the case for the hippocampus (Table 2b). Only subthreshold explorations at p < 0.005 uncorrected in the ROIs, suggested that MDD was associated with lower GM volume in clusters in the IFG and ACC (Table 2b).
Table 2B.
Results for SPM whole brain analyses for gray and white matter volumes; and for small volume corrected ROIs, which passed the initial threshold, but were not significant after FWE-cluster correction.
| MNI-coordinate peak |
p-Value FWE-cluster corrected |
|||||||
|---|---|---|---|---|---|---|---|---|
| Comparison | Region | k | x | y | z | F-peak | Z-peak | |
| Whole brain | ||||||||
| HT ∗ MDD | MCC | 2247 | 9 | −31 | 45 | 25.1 | 4.75 | .011 |
| HT ∗ MDD | Cerebellum | 2138 | −28 | −69 | −50 | 13.6 | 3.37 | .014 |
| Small volume corrected | ||||||||
| MDD− > MDD+ | IFG | 56 | −52 | 27 | 30 | 12.07 | 3.24 | .740 |
| MDD− > MDD+ | 31 | 54 | 17 | 0 | 9.44 | 2.83 | .811 | |
| MDD− > MDD+ | ACC | 241 | −2 | 38 | 10 | 11.07 | 3.09 | .370 |
| HT ∗ MDD | 467 | −3 | 36 | 10 | 13.58 | 3.45 | .163 | |
| HT ∗ MDD | Hippocampus | 13 | −14 | −4 | −21 | 9.29 | 2.80 | .868 |
FWE-cluster corrected p < .05 at initial threshold p < .005; k > 50. GM = gray matter; HT = hypertension; MDD = major depressive disorder; MCC = mid-cingulate cortex; IFG = inferior frontal gyrus; ACC = anterior cingulate cortex; k = cluster size; MNI-coordinate = coordinates of the voxel showing peak significance in mean MNI-space (defined by the Montreal Neurological Institute). Comparisons in the analyses: main effects of HT and MDD and interaction effects of HT ∗ MDD, adjusted for scan location, sex, age and anxiety. HT ∗ MDD = interaction effect; MDD− > MDD+ = lower GM for participants with MDD.
Furthermore, whole-brain voxel-based analyses (Table 2b) revealed significant interaction effects in the posterior mid-cingulate cortex (MCC) (k = 2247; F = 25.1; pFWE = 0.01) and in the cerebellum (k = 2138; F = 13.6; pFWE = 0.01) (Fig. 2 and Table 2b). Fig. 3A shows a similar pattern for the MCC as was found for the ACC, in which MDD in the presence, but not in the absence of hypertension shows a lower GM volume. Stratifying the effect of MDD on the presence and absence of hypertension, confirmed this observation. Namely, MDD in the presence of hypertension was associated with significantly less GM volume in clusters located in the MCC (pFWE = 0.003; k = 3526; T = 4.55); there was no effect of depression in the absence of hypertension (pFWE = 0.93), neither a subthreshold effect (cluster level puncorr > 0.05). Furthermore, Fig. 3A shows that the comorbid group was associated with the lowest MCC volume of all groups.
Fig. 2.
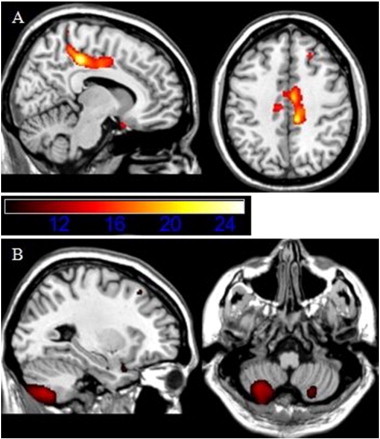
Whole brain interaction effects of hypertension and MDD in A. the posterior mid-cingulate cortex (MCC) and B. the cerebellum. Images are presented in neurological convention. Color intensities reflect F-values.
Fig. 3.
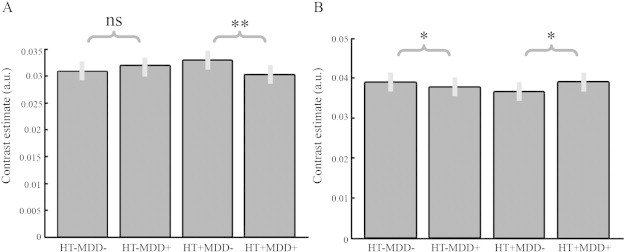
Pairwise comparison of voxel-based GM volumes in the posterior MCC (A) and cerebellum (B). HT = hypertension; MDD = major depressive disorder. **: significantly different in the FWE-corrected t-test; *: significantly different in uncorrected t-test; ns: not significant.
For the cerebellum, a different pattern was observed (Fig. 3B); MDD in the presence of hypertension seemed to be associated with an increased volume in the cerebellum, while MDD in the absence of hypertension seemed to be associated with a decreased volume. Stratifying the effect of MDD on the presence and absence of hypertension showed only subthreshold effects. MDD in the presence of hypertension was associated with subthreshold increased GM volume (cluster level puncorr = 0.05; k = 727; T = 3.01), and MDD in the absence of hypertension was associated with subthreshold decreased GM volume (cluster level puncorr = 0.01; k = 1202; T = 3.44). Whole-brain voxel-based analyses did not reveal significant main effects for hypertension, nor for MDD.
Furthermore, participants with hypertension were on average 6 years older compared to participants without hypertension. In order to exclude the possibility of a confounding effect of age, we age-matched the hypertension and non-hypertension groups by randomly excluding 60 younger individuals from the non-hypertension groups. However, this did not substantially affect the results (data not shown).
4. Discussion
In this structural imaging study we examined whether hypertension and MDD are associated with volumetric differences in overlapping brain areas, and whether effects are independent or interactive. There were no independent effects for hypertension and MDD for gray and white matter volumes. However, we did observe an interaction effect for GM volumes in areas implicated in the regulation of emotional and autonomic functions: the ACC and in the posterior MCC. This interaction indicated that only depression in the presence, but not in the absence of hypertension was associated with lower volumes. Persons with comorbid hypertension and MDD had the lowest GM in these areas compared to the other groups. Furthermore, an interaction effect for GM volume in the cerebellum was observed, which showed the opposite pattern; depression in the presence of hypertension was associated with marginally higher volumes, whereas depression in the absence of hypertension was associated with marginally lower volumes. These findings suggest that MDD is differently associated with brain volumes in the presence or absence of hypertension.
4.1. Interaction effects
The most important finding of this study was the interaction effect of hypertension and MDD on GM volumes in the ACC and MCC. Brain volumes in these areas have previously been associated with hypertension and depression in studies examining these conditions separately (Arnone et al., 2012; Beauchet et al., 2013; Bora et al., 2012; Du et al., 2012; Gianaros et al., 2006; Jennings et al., 2012; Jennings and Zanstra, 2009; Lai, 2013; Maillard et al., 2012; Raz et al., 2003). The ACC and MCC were previously found to be involved in a variety of emotional and cognitive processes, but also in autonomic functions such as cardiovascular modulation (Critchley et al., 2011; Gasquoine, 2013; Parvizi et al., 2013; Williamson et al., 2006). Stressor-evoked blood pressure reactivity has been associated with lower gray matter volume and increased activation in the ACC (Gianaros et al., 2008). The ACC is part of the central autonomic network (CAN), in which the sympathetic output is under tonic inhibitory control (Thayer and Lane, 2007). Disruption of this inhibitory pathway may result in increased heart rate and decreased HRV, as well as hypertension (Thayer and Lane, 2007).
According to the allostatic load theory (McEwen, 2006), prolonged periods of psychosocial stress (often occurring in depression) can lead to chronic frontal hypoactivity and sympathetic disinhibition, increasing the risk for cardiovascular diseases. In line with this, prolonged stress has been associated with damage to brain structures including the ACC (Baune et al., 2012; Brotman et al., 2007; Grippo and Johnson, 2002; Harrison et al., 2013). Besides the cardiovascular system, the ACC is also part of the emotional and stress networks (Gianaros et al., 2005; Phillips et al., 2003). Considering its role in emotion, the ACC has been implicated in monitoring emotional salience (pleasant/averseness) and initiating changes in behavior in reaction to challenging physical and cognitive states (Gasquoine, 2013; Parvizi et al., 2013). Taken together, findings of previous studies and the current findings of the interaction effect of depression and hypertension on cingulate cortex volumes, support the hypothesis that abnormalities in these regions may be one of the explanatory factors for the comorbidity of cardiovascular problems and depression (Gilbert et al., 2010). Multimodal neuroimaging, examining structure and function concurrently, may give more insight into the mechanisms underlying the previous and current findings for the ACC. Although this cross-sectional study precludes inferences regarding causality, it may be that the identified brain volumetric differences are predisposed to MDD and hypertension, whether or not due to prolonged stress exposure.
Interestingly, the current study also showed an interaction effect of hypertension and depression in the cerebellum. The cerebellum is not only involved in motor coordination but is also suggested to play a role in cognitive and emotional functioning (Baumann and Mattingley, 2012). However, the current findings may be spurious, as the unexpected pattern of increased volume of the cerebellum that was observed for MDD in the presence of hypertension, and decreased volume for MDD without hypertension, were no longer statistically significant after adjustment for multiple testing. Nevertheless, as the cluster size was substantial, we cannot as yet discard this finding.
4.2. Integrating findings with existing theories on depression subtypes
In line with existing theories (Kendler et al., 2009; Alexopoulos et al., 1997; Ormel and de Jonge, 2011), persons with comorbid depression and hypertension differed on a number of characteristics with respect to the comparison groups. The comorbid group had a higher age of depression onset, and a higher severity of depressive symptoms than the group of depressed participants without hypertension. This could not explain the GM findings in the cingulate cortex, since depression severity was not associated with GM volumes, whereas a higher age of depression onset was associated with larger instead of smaller volumes (van Tol et al., 2010). On the other hand, the comorbid group was associated with a lower average systolic blood pressure compared to hypertension without depression, despite the observed lower GM volume for this group. Furthermore, metabolic syndrome was twice more prevalent in the comorbid group compared to the hypertension group without depression. Including metabolic syndrome as an additional covariate did not substantially change the results. Nonetheless, GM volumetric abnormalities may predispose towards a broader array of cardiovascular and metabolic alterations, surpassing hypertension (Onyewuenyi et al., 2014). Thus, our findings support the idea that the presence of comorbidity between depression and hypertension may indicate a vulnerability that can be visualized in specific brain structures.
Another possibility is that persons with comorbid hypertension and MDD are more vulnerable to develop regional brain abnormalities than persons with either one condition. This might explain why in this young subclinical sample associations were only visible in patients with comorbid MDD and hypertension, and not (yet) in patients with hypertension or MDD only. In addition, the findings are consistent with the vascular depression hypothesis, in which a subtype of depression is postulated with specific structural brain alterations in areas of emotion regulation, as a result of vascular pathology (Alexopoulos et al., 1997; Thomas et al., 2002). Thus, the current findings are in line with several theories, which do not have to be mutually exclusive.
4.3. Absence of independent effects
We hypothesized to find lower brain volumes for hypertension and MDD in overlapping brain regions. Unexpectedly, the current study found no independent effects for either hypertension or MDD. The current sample may differ from previous hypertension samples, because participants with systolic blood pressure >180 mm Hg and/or diastolic blood pressure >120 mm Hg and participants using beta-blockers potentially influencing brain hemodynamics were excluded from the current study. Also, the relatively young age (mean age = 42) of the participants with hypertension in this sample, and the short duration of the self-reported hypertension (mean age at onset = 38) may be relevant factors, as GM volume reductions observed in participants with high blood pressure may aggravate with increasing age (Beauchet et al., 2013). Nevertheless, in another large study of young individuals (mean age = 39), high blood pressure was correlated with lower GM volumes in the temporal lobe (Maillard et al., 2012). Considering the current tentative findings, future research should focus on the nature of volumetric differences in early and prodromal stages of disease.
As previous studies on hypertension and MDD independently reported lower brain volumes in frontal-limbic brain areas, we selected the hippocampus, ACC and IFG as ROIs. We only observed significant effects of MDD in the ACC. Of note, a previous study in the same sample found that depression was associated with lower volumes in clusters in the IFG and ACC, compared to healthy controls (van Tol et al., 2010). However, in our analyses we used a more stringent threshold, patients with anxiety without comorbid MDD were pooled within the control group, and anxiety was taken into account by including it as covariate instead of analyzing it as an isolated group. Since anxiety was equally distributed across the four groups in the present study, it could not influence the interaction findings. Nevertheless, in the FWE-uncorrected analyses, MDD was associated with clusters of reduced GM volume in the IFG and ACC.
The current study found no main or interaction effects for depression and hypertension for WM volume analyses. So far, the association between WM volumes and depression has not been studied extensively and results have been inconsistent (Abe et al., 2010; Kim et al., 2008; Steingard et al., 2002). One volumetric MRI study found that blood pressure did not correlate with regional WM volumes (Gianaros et al., 2006). Furthermore, research on the effect of hypertension on WM has focused predominantly on WM lesions and WM integrity instead of WM volumes. One study found that after 50 years of age, but not before, comorbid hypertension, obesity, and diabetes mellitus were associated with significantly larger WM lesion volumes compared to an age matched group without these conditions (King et al., 2014).
4.4. Strengths and limitations
Our findings should be considered in light of the following strengths and limitations. The first major strength is that this is the first study that examined the neural correlates of hypertension and depression in one sample. Another strength of this study is that the size of the sample was sufficient to test for interaction effects of hypertension and MDD. Furthermore, a strength is the use of a diagnostic interview to assess depression. A limitation is that blood pressure was only measured twice at one visit, which might not indicate chronic high blood pressure. Another limitation is that the use of particular types of beta-blockers, which are commonly used in the treatment of hypertension, was an exclusion criterion, making the findings potentially less generalizable to a more clinical population.
5. Conclusion
In the current study hypertension and MDD were not independently, but only interactively associated with altered GM volumes. The results suggest that in a relatively young sample, the combination of MDD and hypertension is uniquely associated with lower brain volumes in areas implicated in the regulation of emotional and autonomic functions. The observed interaction effect implies that future volumetric neuroimaging studies in depression should take into account the presence of comorbid hypertension. Moreover, further research should be undertaken to replicate these findings in a sample with more advanced vascular disease. In addition, longitudinal research is needed to clarify whether comorbid vascular disease and MDD may be a subgroup with specific brain abnormalities, and/or whether hypertension and MDD reinforce each other's impact on brain volumes.
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Acknowledgments
The infrastructure for the NESDA study (http://www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organisation for Health Research and Development (Zon-Mw, grant number 10-000-1002) and is supported by the participating universities and mental health care organizations: VU University Medical Center, GGZ inGeest, Arkin, Leiden University Medical Center, GGZ Rivierduinen, University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Scientific Institute for Quality of Healthcare (IQ healthcare), Netherlands Institute for Health Services Research (NIVEL) and Netherlands Institute of Mental Health and Addiction (Trimbos Institute). The present study was supported by a VIDI grant from the Dutch Medical Research Council (grant 016.086.397).
Contributor Information
Maaike Meurs, Email: a.m.roest@umcg.nl.
Nynke A. Groenewold, Email: n.a.groenewold01@umcg.nl.
Annelieke M. Roest, Email: a.m.roest@umcg.nl.
Nic J.A. van der Wee, Email: N.J.A.van_der_wee@lumc.nl.
Dick J. Veltman, Email: dj.veltman@vumc.nl.
Marie-José van Tol, Email: m.j.van.tol@umcg.nl.
Peter de Jonge, Email: peter.de.jonge@umcg.nl.
References
- Abe O., Yamasue H., Kasai K., Yamada H., Aoki S., Inoue H., Takei K., Suga M., Matsuo K., Kato T., Masutani Y., Ohtomo K. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry Res. 2010;181(1):64–70. doi: 10.1016/j.pscychresns.2009.07.007. 19959342 [DOI] [PubMed] [Google Scholar]
- Alexopoulos G.S., Meyers B.S., Young R.C., Campbell S., Silbersweig D., Charlson M. ‘Vascular depression’ hypothesis. Arch. Gen. Psychiatry. 1997;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. 9337771 [DOI] [PubMed] [Google Scholar]
- Arnone D., McIntosh A.M., Ebmeier K.P., Munafò M.R., Anderson I.M. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur. Neuropsychopharmacol. 2012;22(1):1–16. doi: 10.1016/j.euroneuro.2011.05.003. 21723712 [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. 17761438 [DOI] [PubMed] [Google Scholar]
- Baumann O., Mattingley J.B. Functional topography of primary emotion processing in the human cerebellum. Neuroimage. 2012;61(4):805–811. doi: 10.1016/j.neuroimage.2012.03.044. 22465459 [DOI] [PubMed] [Google Scholar]
- Baune B.T., Stuart M., Gilmour A., Wersching H., Heindel W., Arolt V., Berger K. The relationship between subtypes of depression and cardiovascular disease: a systematic review of biological models. Transl. Psychiatry. 2012;2:e92. doi: 10.1038/tp.2012.18. 22832857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O., Celle S., Roche F., Bartha R., Montero-Odasso M., Allali G., Annweiler C. Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J. Hypertens. 2013;31(8):1502–1516. doi: 10.1097/HJH.0b013e32836184b5. 23811995 [DOI] [PubMed] [Google Scholar]
- Bora E., Fornito A., Pantelis C., Yücel M. Gray matter abnormalities in Major depressive disorder: a meta-analysis of voxel based morphometry studies. J. Affect. Disord. 2012;138(1–2):9–18. doi: 10.1016/j.jad.2011.03.049. 21511342 [DOI] [PubMed] [Google Scholar]
- Brotman D.J., Golden S.H., Wittstein I.S. The cardiovascular toll of stress. Lancet. 2007;370(9592):1089–1100. doi: 10.1016/S0140-6736(07)61305-1. 17822755 [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Nagai Y., Gray M.A., Mathias C.J. Dissecting axes of autonomic control in humans: insights from neuroimaging. Auton. Neurosci. 2011;161(1–2):34–42. doi: 10.1016/j.autneu.2010.09.005. 20926356 [DOI] [PubMed] [Google Scholar]
- de Jonge P., Rosmalen J.G., Kema I.P., Doornbos B., van Melle J.P., Pouwer F., Kupper N. Psychophysiological biomarkers explaining the association between depression and prognosis in coronary artery patients: a critical review of the literature. Neurosci. Biobehav. Rev. 2010;35(1):84–90. doi: 10.1016/j.neubiorev.2009.11.025. 19962401 [DOI] [PubMed] [Google Scholar]
- Du M.Y., Wu Q.Z., Yue Q., Li J., Liao Y., Kuang W.H., Huang X.Q., Chan R.C., Mechelli A., Gong Q.Y. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;36(1):11–16. doi: 10.1016/j.pnpbp.2011.09.014. 22001316 [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. 11368702 [DOI] [PubMed] [Google Scholar]
- Gasquoine P.G. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci. Biobehav. Rev. 2013;37(3):340–348. doi: 10.1016/j.neubiorev.2013.01.002. 23313645 [DOI] [PubMed] [Google Scholar]
- Gianaros P.J., Derbyshire S.W., May J.C., Siegle G.J., Gamalo M.A., Jennings J.R. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42(6):627–635. doi: 10.1111/j.1469-8986.2005.00366.x. 16364058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P.J., Greer P.J., Ryan C.M., Jennings J.R. Higher blood pressure predicts lower regional grey matter volume: consequences on short-term information processing. Neuroimage. 2006;31(2):754–765. doi: 10.1016/j.neuroimage.2006.01.003. 16488626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P.J., Sheu L.K., Matthews K.A., Jennings J.R., Manuck S.B., Hariri A.R. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J. Neurosci. 2008;28(4):990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. 18216206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert A.M., Prasad K., Goradia D., Nutche J., Keshavan M., Frank E. Grey matter volume reductions in the emotion network of patients with depression and coronary artery disease. Psychiatry Res. 2010;181(1):9–14. doi: 10.1016/j.pscychresns.2009.07.006. 19959343 [DOI] [PubMed] [Google Scholar]
- Grippo A.J., Johnson A.K. Biological mechanisms in the relationship between depression and heart disease. Neurosci. Biobehav. Rev. 2002;26(8):941–962. doi: 10.1016/s0149-7634(03)00003-4. 12667498 [DOI] [PubMed] [Google Scholar]
- Groenewold N.A., Opmeer E.M., de Jonge P., Aleman A., Costafreda S.G. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 2013;37(2):152–163. doi: 10.1016/j.neubiorev.2012.11.015. 23206667 [DOI] [PubMed] [Google Scholar]
- Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A., Gordon D.J., Krauss R.M., Savage P.J., Smith S.C., Jr, Spertus J.A., Costa F., American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: executive summary. Crit. Pathw. Cardiol. 2005;4:198–203. doi: 10.1097/00132577-200512000-00018. 16157765 [DOI] [PubMed] [Google Scholar]
- Harrison N.A., Cooper E., Voon V., Miles K., Critchley H.D. Central autonomic network mediates cardiovascular responses to acute inflammation: relevance to increased cardiovascular risk in depression? Brain Behav. Immun. 2013;31:189–196. doi: 10.1016/j.bbi.2013.02.001. 23416033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings J.R., Mendelson D.N., Muldoon M.F., Ryan C.M., Gianaros P.J., Raz N., Aizenstein H. Regional grey matter shrinks in hypertensive individuals despite successful lowering of blood pressure. J. Hum. Hypertens. 2012;26(5):295–305. doi: 10.1038/jhh.2011.31. 21490622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings J.R., Zanstra Y. Is the brain the essential in hypertension? Neuroimage. 2009;47(3):914–921. doi: 10.1016/j.neuroimage.2009.04.072. 19410005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Fiske A., Gardner C.O., Gatz M. Delineation of two genetic pathways to major depression. Biol. Psychiatry. 2009;65(9):808–811. doi: 10.1016/j.biopsych.2008.11.015. 19103442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Ustün T.B. The World Mental Health (WMH) survey initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int. J. Methods Psychiatr. Res. 2004;13(2):93–121. doi: 10.1002/mpr.168. 15297906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Hamilton J.P., Gotlib I.H. Reduced caudate gray matter volume in women with major depressive disorder. Psychiatry Res. 2008;164(2):114–122. doi: 10.1016/j.pscychresns.2007.12.020. 18930633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King K.S., Peshock R.M., Rossetti H.C., McColl R.W., Ayers C.R., Hulsey K.M., Das S.R. Effect of normal aging versus hypertension, abnormal body mass index, and diabetes mellitus on white matter hyperintensity volume. Stroke. 2014;45(1):255–257. doi: 10.1161/STROKEAHA.113.003602. 24203844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabbers G., Bosma H., Van der Does A.J., Vogelzangs N., Kempen G.I., Van Eijk J.T., Penninx B.W. The educational patterning of health-related adversities in individuals with major depression. J. Affect. Disord. 2010;126(1–2):96–102. doi: 10.1016/j.jad.2010.02.128. 20299107 [DOI] [PubMed] [Google Scholar]
- Krishnan K.R., Taylor W.D., McQuoid D.R., MacFall J.R., Payne M.E., Provenzale J.M., Steffens D.C. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol. Psychiatry. 2004;55(4):390–397. doi: 10.1016/j.biopsych.2003.08.014. 14960292 [DOI] [PubMed] [Google Scholar]
- Lai C.H. Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry Res. 2013;211(1):37–46. doi: 10.1016/j.pscychresns.2012.06.006. 23146253 [DOI] [PubMed] [Google Scholar]
- Maillard P., Seshadri S., Beiser A., Himali J.J., Au R., Fletcher E., Carmichael O., Wolf P.A., DeCarli C. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol. 2012;11(12):1039–1047. doi: 10.1016/S1474-4422(12)70241-7. 23122892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Protective and damaging effects of stress mediators: central role of the brain. Dial. Clin. Neurosci. 2006;8(4):367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. 17290796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L., Chen D., Yang Y., Zheng Y., Hui R. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J. Hypertens. 2012;30(5):842–851. doi: 10.1097/HJH.0b013e32835080b7. 22343537 [DOI] [PubMed] [Google Scholar]
- Nemeroff C.B., Goldschmidt-Clermont P.J. Heartache and heartbreak — the link between depression and cardiovascular disease. Nat. Rev. Cardiol. 2012;9(9):526–539. doi: 10.1038/nrcardio.2012.91. 22733213 [DOI] [PubMed] [Google Scholar]
- Onyewuenyi I.C., Muldoon M.F., Christie I.C., Erickson K.I., Gianaros P.J. Basal ganglia morphology links the metabolic syndrome and depressive symptoms. Physiol. Behav. 2014;123:214–222. doi: 10.1016/j.physbeh.2013.09.014. 24096008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J., de Jonge P. Unipolar depression and the progression of coronary artery disease: toward an integrative model. Psychother. Psychosom. 2011;80(5):264–274. doi: 10.1159/000323165. 21646821 [DOI] [PubMed] [Google Scholar]
- Parvizi J., Rangarajan V., Shirer W.R., Desai N., Greicius M.D. The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron. 2013;80(6):1359–1367. doi: 10.1016/j.neuron.2013.10.057. 24316296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx B.W., Beekman A.T., Smit J.H., Zitman F.G., Nolen W.A., Spinhoven P., Cuijpers P., De Jong P.J., Van Marwijk H.W., Assendelft W.J., Van Der Meer K., Verhaak P., Wensing M., De Graaf R., Hoogendijk W.J., Ormel J., Van Dyck R., NESDA Research Consortium The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int. J. Methods Psychiatr. Res. 2008;17(3):121–140. doi: 10.1002/mpr.256. 18763692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. 12946879 [DOI] [PubMed] [Google Scholar]
- Pickering T.G., Hall J.E., Appel L.J., Falkner B.E., Graves J.W., Hill M.N., Jones D.H., Kurtz T., Sheps S.G., Roccella E.J., Council on High Blood Pressure Research Professional and Public Education Subcommittee. American Heart Association Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J. Clin. Hypertens. (Greenwich) 2005;7(2):102–109. doi: 10.1111/j.1524-6175.2005.04377.x. 15722655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N., Rodrigue K.M., Acker J.D. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav. Neurosci. 2003;117(6):1169–1180. doi: 10.1037/0735-7044.117.6.1169. 14674838 [DOI] [PubMed] [Google Scholar]
- Ridgway G.R., Omar R., Ourselin S., Hill D.L., Warren J.D., Fox N.C. Issues with threshold masking in voxel-based morphometry of atrophied brains. Neuroimage. 2009;44(1):99–111. doi: 10.1016/j.neuroimage.2008.08.045. 18848632 [DOI] [PubMed] [Google Scholar]
- Steingard R.J., Renshaw P.F., Hennen J., Lenox M., Cintron C.B., Young A.D., Connor D.F., Au T.H., Yurgelun-Todd D.A. Smaller frontal lobe white matter volumes in depressed adolescents. Biol. Psychiatry. 2002;52(5):413–417. doi: 10.1016/s0006-3223(02)01393-8. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Lane R.D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 2007;74(2):224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Thomas A.J., O’Brien J.T., Davis S., Ballard C., Barber R., Kalaria R.N., Perry R.H. Ischemic basis for deep white matter hyperintensities in major depression. Arch. Gen. Psychiatry. 2002;59(9):785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- van Tol M.J., van der Wee N.J.A., van den Heuvel O.A., Nielen M.M.A., Demenescu L.R., Aleman A., Renken R., van Buchem M.A., Zitman F.G., Veltman D.J. Regional brain volume in depression and anxiety disorders. Arch. Gen. Psychiatry. 2010;67(10):1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- Wardenaar K.J., van Veen T., Giltay E.J., den Hollander-Gijsman M., Penninx B.W.J.H., Zitman F.G. The structure and dimensionality of the inventory of depressive symptomatology self report (IDS-SR) in patients with depressive disorders and healthy controls. J. Affect. Disord. 2010;125(1–3):146–154. doi: 10.1016/j.jad.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Williamson J.W., Fadel P.J., Mitchell J.H. New insights into central cardiovascular control during exercise in humans: a central command update. Exp. Physiol. 2006;91(1):51–58. doi: 10.1113/expphysiol.2005.032037. [DOI] [PubMed] [Google Scholar]
- Woo M.A., Kumar R., Macey P.M., Fonarow G.C., Harper R.M. Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J. Card. Fail. 2009;15(3):214–223. doi: 10.1016/j.cardfail.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


