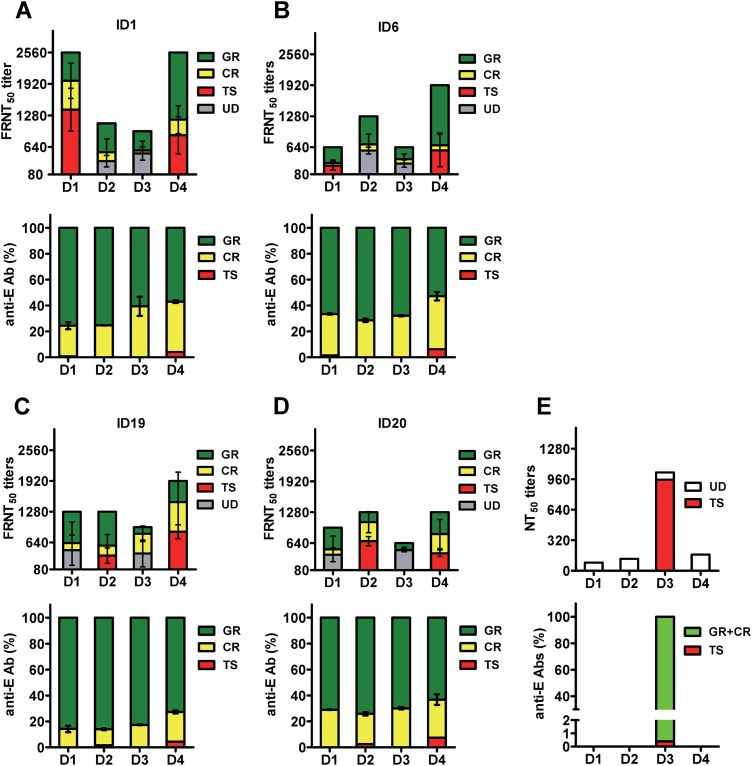
FIG 7.
Composition of anti-E Abs and contribution of different anti-E Abs to neutralizing activities in four vaccinees after secondary immunization. (A to D) The proportion of different anti-E Abs (percentages of GR, CR, and TS Abs) were based on data shown in Fig. 5E, and the contributions of GR and CR Abs to neutralizing activities are based on the percent reductions of FRNT50 titers in Table 2 and Fig. 4D for four vaccinees (ID1 [A], ID6 [B], ID19 [C], and ID20 [D]). After depletion of GR and CR Abs, the remaining neutralizing activities to previously nonexposed serotypes were designated undetermined (UD). (E) As a comparison, data from a previously reported case of primary DENV3 infection were summarized. Shown are the percentages of TS anti-E Abs, estimated based on endpoint titers in recombinant DIII and virion ELISAs (43), and their contribution to the monotypic neutralizing activity (27).