Abstract
Pneumococcal infections, including pneumonia and invasive disease, are major sources of morbidity and mortality worldwide. Prevention of the first acquisition of Streptococcus pneumoniae through the use of vaccines represents an effective method to reduce the burden of the disease in both children and adults. Two vaccines are currently available in adults: a pneumococcal polysaccharide vaccine (PPV23) that includes 23 purified capsular polysaccharide antigens and a pneumococcal protein-conjugate vaccine (PCV13) that includes capsular polysaccharide antigens covalently linked to a non-toxic protein. The PPV23 induces a humoral immune response and since it has been licensed has been the subject of debates and controversies. Numerous studies and meta-analyses have shown that PPV23 protects against invasive pneumococcal disease, although there are conflicting data regarding its efficacy for the prevention of pneumonia. Vaccination with PCV13 stimulates good antibody responses as well as mucosal immunity and suppresses colonization. A conjugate vaccine can be expected to have benefits over a polysaccharide vaccine because of the characteristics of a T-cell-dependent response in terms of affinity, maturation of antibodies with repeated exposure, induction of immunological memory and long-lasting immunity. PCV13 has demonstrated all of these characteristics in children and fundamental differences in adults are not expected. The efficacy in adults is currently being investigated and results will be available soon.
Keywords: Invasive pneumococcal disease, pneumococcal, pneumonia, Streptococcus pneumoniae, vaccination, vaccine
Introduction
Infections due to Streptococcus pneumoniae represent one of the major causes of morbidity and mortality worldwide [1,2]. Pneumococcal disease can be broadly grouped into categories of non-invasive pneumococcal disease, also termed mucosal infection (otitis, sinusitis and pneumonia without bacteraemia), and invasive pneumococcal disease (IPD) occurring when the organism invades normally sterile sites, such as bloodstream infection, pneumonia with bacteraemia and infection of the meninges. In subjects with chronic medical conditions, the incidence of IPD rises to 176–483/100 000 persons, and reaches 342–2031/100 000 persons among immunosuppressed patients [3,4]. Pneumococcal infections show a bimodal distribution, with high prevalence in both children and the elderly. The incidence of IPD is affected by a number of factors, including smoking status, immune function, age and geographical location [5]. Even when appropriate antibiotic treatment is instituted, mortality due to IPD remains high, involving 10–25% of patients [6].
Most of the burden of pneumococcal disease in adults is related to pneumonia, which represents a major respiratory tract infection with a high prevalence in the general population and with clinical heterogeneity and variable severity [7,8]. Although the use of antibiotics is necessary for the treatment of pneumococcal infections, the level of antibiotic resistance in S. pneumoniae is increasing and the current development programme for new antibiotics will not provide new drugs in the near future.
Is it possible to improve the management of patients with pneumococcal infections?
It could be argued that both early diagnosis and treatment together represent the cornerstone in the management of pneumococcal infections and they could reduce the burden of pneumococcal disease. However, microbiological diagnosis of S. pneumoniae infection is usually reached in a low percentage of patients with community-acquired pneumonia [9,10].
The identification of S. pneumoniae rarely leads to a change in empiric antibiotic therapy. When a change in antibiotic therapy is necessary, this intervention does not appear to affect the patients’ prognosis, probably because of the latency between the time of the onset of infection and the time of microbiological diagnosis [11].
Streptococcus pneumoniae is part of the commensal flora of the upper respiratory tract and colonizes the nasopharyngeal niche. Colonization seems to be an important feature because pneumococcal disease will not occur without the preceding nasopharyngeal colonization [12]. Streptococcus pneumoniae colonization occurs early in infancy and rapidly spreads among children and family members. For this reason, prevention of first acquisition of S. pneumoniae among children with the use of a vaccine represents an effective method to reduce the infection in both child and adult populations.
The design of a pneumococcal vaccine
The design of a pneumococcal vaccine is an important challenge, with the main concern being the high number of different serotypes. Data from the Tigecycline Evaluation Surveillance Trial (TEST) have shown that among the 92 different serotypes, the most common in children were 19A, 19F and 14, whereas the most common among adults were 19A, 3, 6A and 7F [13]. Some serotypes seem to favour specific organs: serotypes 1 and 3 are more often isolated from the lung during pneumonia, whereas serotypes 6, 10 and 23 are regularly isolated from the meninges during meningitis [14,15]. Approximately 23 serotypes are responsible for 80–90% of IPD [16].
The serotype involved is also associated with severity of disease, and the clinical impact of a pneumococcal vaccination usually depends on the coverage of serotypes frequently associated with invasive disease or antibiotic resistance.
Knowledge of local serotype predominance represents one of the key points for a successful vaccination programme. Serotypes 1, 3, 4, 6A, 6B, 7F, 8, 9V, 14, 18C, 19F and 23F are the dominant serotypes associated with IPD worldwide [17]. However, this distribution may vary significantly between geographic locations.
The most frequent serotypes in Europe are 1, 3, 7F, 14 and 19a, but large differences can be found among countries, as shown in Table 1 [18–25]. A recently published meta-analysis showed that S. pneumoniae serotypes isolated from young children with invasive pneumococcal diseases varied significantly from a geographical point of view. However, serotype 14 was the most common isolate throughout the world, and serotypes 1, 5, 6A, 6B, 14, 19F and 23F were isolated in 50% of individuals [26].
TABLE 1.
Most prevalent serotypes in adult pneumococcal disease among nine European countries
| 1 | 2 | 3 | 4 | 5 | 6A | 6B | 7F | 8 | 9V | 11A | 12F | 14 | 15B | 17F | 18C | 19A | 19F | 22F | 23F | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UK | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Portugal | X | X | X | X | X | X | ||||||||||||||
| Greece | X | X | X | X | X | X | ||||||||||||||
| Spain | X | X | X | X | X | X | X | X | X | |||||||||||
| France | X | X | X | X | X | |||||||||||||||
| Germany | X | X | X | X | X | X | X | X | X | |||||||||||
| Belgium | X | X | X | X | X | X | X | |||||||||||||
| Netherlands | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Switzerland | X | X | X | X | X | X | X | X | X | X |
The four main principles of pneumococcal vaccine design are: (i) cover as many serotypes as possible; (ii) cover the most common serotypes; (iii) cover serotypes associated with severe disease or antibiotic resistance; and (iv) ensure longevity of immunity.
Pneumococcal polysaccharide vaccine
Streptococcus pneumoniae vaccines are based on the use of the capsular polysaccharides, which induce type-specific antibodies that activate and fix complement and promote bacterial opsonization and phagocytosis. The current pneumococcal polysaccharide vaccine stimulates a humoral immune response against the capsular polysaccharide on the surface of the bacteria. The first observation regarding the efficacy of pneumococcal vaccine was in 1911 in South Africa [27]. However, the first advanced pneumococcal vaccine, which included 14 different serotypes, was approved by the US Food and Drug Administration (FDA) in 1977. It was changed to a 23-valent vaccine (PPV23) in 1983 with 25 μg of surface polysaccharide of 23 different serotypes, including 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F and 33F [28]. In the USA, almost 70% of the elderly have been vaccinated with PPV23. This has led to a reduction of IPD in adults. However, no reduction in IPD has been documented in immunocompromised patients, and there is little evidence for reductions in pneumococcal pneumonia [29]. Furthermore, the effectiveness of PPV23 seems to decrease with both age and time since vaccination, probably because of a weak and short-lasting IgM immune response, the absence of affinity maturation and antibody class switching to IgG. A lack of booster effect of re-vaccination limits the effectiveness of vaccination to only a few years [30,31].
Recently, a Cochrane meta-analysis showed that PPV23 is slightly effective in reducing IPD in healthy adults, but the evidence for its effectiveness against all-cause mortality in patients with pneumonia was inconclusive. Furthermore, vaccine effectiveness in adults with chronic illness and with immunosuppression appeared poor [32]. In 2008, WHO indicated the need for a more effective conjugate vaccine or other type of vaccines covering the pneumococcal serotypes causing serious illness in both children and adults [33].
Pneumococcal conjugate vaccines
Conjugate vaccines seem to produce a stronger immune response because of the combination of polysaccharide protein antigen conjugate that is captured by B cells and other antigen-presenting cells presenting antigens to T helper type 2 (Th2) cells. The activation of Th2 leads B cells to differentiate into memory B cells and plasma cells. This results in a more rapid production of antibodies in comparison to the switch following primary antigen exposure [34]. Conjugate vaccines have been used in children as well as in adults with defective immune systems to promote a more robust immune response.
PCV7 is a conjugate pneumococcal vaccine that includes fewer serotypes than PPV23: serotypes in PCV7 are 4, 6B, 9V, 14, 18C, 19F and 23F. Its introduction in US children has reduced the incidence of IPD from >200 cases/100 000 persons to >50 cases/100 000 persons with a significant herd effect in the incidence of IPD in adults [35,36]. PCV7 vaccination has reduced the annual incidence of IPD and pneumonia due to S. pneumoniae among children younger than 5 years by 97% for serotypes included in the vaccine, but increased the incidence of infection due to non-vaccine serotypes by 22% [37,38]. PCV7, but not PPV23, has been shown to reduce IPD incidence in human immunodeficiency virus (HIV)-infected adults [39]. Finally, PCV7 vaccination seems to be more effective than PPV23 in adults in eliciting an opsonophagocytic activity response at levels that are considered protective in children. However, real effects in reducing IPD and pneumonia incidence in adults have not been demonstrated [40]. PCV10 is a conjugate pneumococcal vaccine that includes the serotypes covered by PCV7 plus serotypes 1, 5 and 7F. The use of PCV10 in newborns resulted in a reduction of the first episodes of otitis media in 34% of patients [41].
PCV13 is a conjugate pneumococcal vaccine that includes the serotypes covered by PCV10 plus serotypes 3, 6A (responsible for IPD) and 19A (responsible for antibiotic resistance). The FDA licensed PCV13 for the prevention of IPD and otitis media in infants and young children in February 2010. In December 2011, the FDA licensed this vaccine for prevention of pneumonia and IPD in people aged ≥50 years [42]. The use of PCV13 in children has decreased the incidence of IPD by 36% and decreased IPD due to serotype 19A by 45% (Kaplan SL, IDSA Annual Meeting 2011. Presentation LB1). A recent survey among the adult population in Israel showed that serotypes included in PCV7 and PCV13 matched 25.6% and 63.7% of pneumococcal serotype isolates and matched 30% and 68% of serotypes in fatal cases, respectively; demonstrating that the introduction of an adult vaccination programme with PCV13 could be useful in reducing pneumococcal infections [43]. The effectiveness of a PCV13 vaccination in adults could be reduced by the increase in the incidence of other serotypes not covered by this vaccine, as happened after the introduction of PCV7 vaccination in children. The occurrence of this effect became evident only after a considerable period of time. PCV13 was introduced in the USA in 2010 and trends in serotypes are not yet available. Finally, the use of a pneumococcal vaccination programme based on PCV13 may be cost effective according to several predictive models [44–46].
Suggestion for the use of pneumococcal vaccines
A large, randomized, placebo-controlled, clinical trial evaluating the efficacy of PCV13 against non-bacteraemic community-acquired pneumonia and IPD is in progress and results will be available in 2014 [47]. However, there is some evidence for the effectiveness of PPV13 in preventing pneumococcal-related disease in adults. Preliminary data on the immune response to PCV13 in adults are not yet available. However, the indications for the use of PCV13 alone and in combination with PPV23 could be suggested. Vaccination with PPV23 is indicated in adults >65 years in different countries, but the protective effect against pneumococcal infection declines rapidly and is inconsistent 3–5 years after the vaccination [48].
A randomized, controlled study enrolling 831 vaccine-naive, immunocompetent adults aged 60–64 years and 403 adults aged 50–59 years, showed that the response to 8 of 12 serotypes included in both vaccines was higher in subjects vaccinated with PPV13 and in the younger cohort. The opsonophagocytic activity declined from the first month to 1 year after vaccination, but remained higher than baseline titres in PCV13. Future pneumococcal vaccination programmes could use PCV13 to reach a more robust immune response and vaccinate people younger than 65 to prevent vaccination failure due to immunosenescence [49].
Recommendations for adult pneumococcal vaccination suggest a second dose of PPV23 after 5 years to overcome the loss of immune protection, but data regarding the effectiveness of this approach are lacking. It could be argued that re-vaccination with PPV23 might not be useful to protect people against pneumococcal infection 5 years after the first dose [50]. The advisory Committee on Immunization Practices recently published a new recommendation for PCV13 [42]. They recommended PCV13 for adults >19 years old with certain underlying disorders, including nephritic syndrome, chronic renal failure, solid organ transplant, generalized malignancy, HIV infection, iatrogenic immunodeficiency (such as systemic corticosteroids), primary immunodeficiencies, asplenia or sickle cell disease.
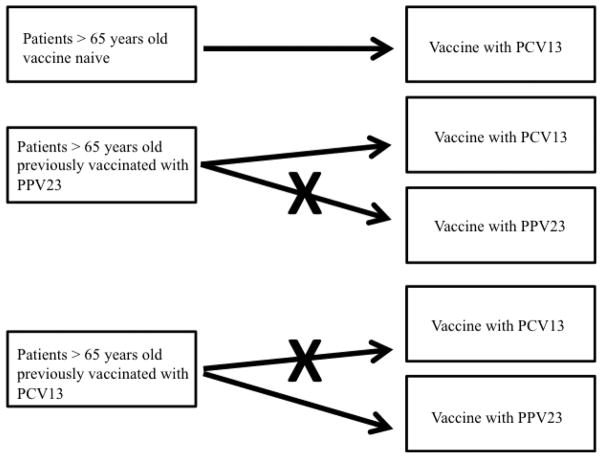
Re-vaccination with PCV13 could be an option for people vaccinated with PPV23 to extend the protection against pneumococcal infection. A preliminary, randomized, controlled study enrolled 900 subjects >70 years who received a second dose of vaccination with PCV13 or PPV23 after receiving PCV13 or PPV23. In adults previously vaccinated with PPV23, PCV13 elicited a statistically significantly greater opsonophagocytic activity response for the majority of serotypes compared with a second vaccination with PPV23. The responses to PCV13 given after PPV23 were significantly lower for all 13 serotypes compared with the initial PCV13 dose, indicating that PPV23 diminished the response to subsequent PCV13. The responses after PCV13/PPV23 were significantly greater for 11 of 12 common serotypes compared with PPV23/PCV13. This suggests that in adults who have already received PPV23, there may be advantages to subsequent administration of PCV13 rather than revaccination with PPV23. A sub-analysis of different age groups in this study showed that immune responses to PCV13 in elderly people between 70–74 and 75–79 years of age were largely similar and slightly lower in those aged 80 years and older [50]. The administration of a second dose of PPV23 to subjects who had received PPV23 3.5–4 years before has led to notably lower immune responses than those observed following the initial PPV23 [51]. However, a second dose of PCV13 produced a response similar to the first dose, and subjects who received PCV13 as first vaccine show a PPV23 response greater than that due to a single dose of PPV23. These results suggest a possible vaccination combination of PCV13 first and PPV23 second with the aim being to obtain a prolonged and strong immune protection against pneumococcal infection in people older than 65 years. An indication for the use of PCV13 and PPV23 in adults is shown in Fig. 1.
FIG. 1.
PCV13 and PPV23: suggestions for pneumococcal vaccine use in immunocompetent patients. PCV13, pneumococcal 13-valent conjugate vaccine; PPV23, pneumococcal polysaccharide 23-valent vaccine.
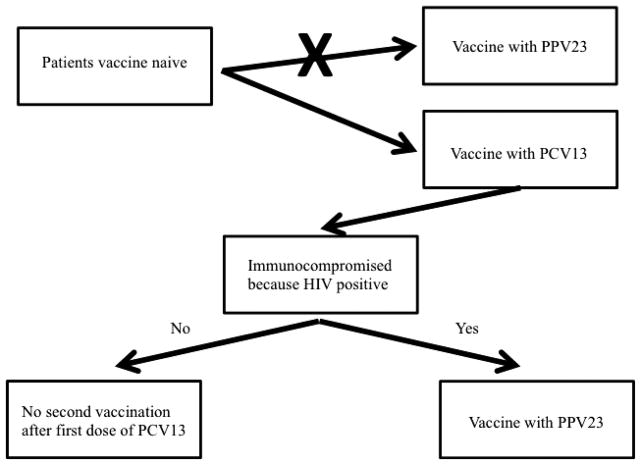
The presence of immunosuppression represents one of the possible indications for pneumococcal vaccination. A study performed in France among HIV-infected patients showed that serotypes 19A, 14, 7F and 6A were the most frequent and S. pneumoniae isolates demonstrated higher levels of resistance to β-lactams and cotrimoxazole. The PPV23 and PCV13 vaccines would have theoretically covered 78% and 70% of the cases, respectively, potentially preventing difficult-to-treat S. pneumoniae infections [52]. In HIV-infected people a vaccination regimen with PCV13, followed by PPV23, is clinically and economically useful because of high pneumococcal disease rates, increasing life expectancy and immune function maintenance by antiretroviral therapy. However, in people with other immunocompromising conditions associated with shorter life expectancy and poorer immune responsiveness, adding PPV23 to PCV13 may be of less benefit [53]. A suggestion for the use of PCV13 and PPV23 in immunocompromised people is shown in Fig. 2.
FIG. 2.
PCV13 and PPV23: suggestion for pneumococcal vaccine use in immunocompromised patients. PCV13, pneumococcal 13-valent conjugate vaccine; PPV23, pneumococcal polysaccharide 23-valent vaccine; HIV, human immunodeficiency virus.
Conclusions
The new conjugate vaccine, PCV13, may provide an opportunity to reduce the burden of pneumococcal disease in both children and adults. PCV13 seems to have a better balance between serotype coverage and immunogenicity. Among adults, PPV23 should not be offered as the first vaccination because of the lack of immunogenicity in the 3–5 years after the vaccination, the lack of booster effect with a second dose, and because of a reduction of immunogenicity effect from PCV13. However, PPV23 seems to be useful as a second vaccination 8 weeks after a PCV13 dose in order to increase serotype coverage. Among immunocompromised patients, PCV13 seems to be superior to PPV23 with regard to immunogenic effects as well as cost effectiveness. This scenario could be applicable not only in HIV-infected populations, but also in other immunocompromised patients. So far, available data are mainly based on the immunogenicity of the different vaccines. More clinical data focused on the effectiveness of vaccination for the reduction of pneumococcal disease will be available in 2014 and may help with further evidence-based recommendations.
Footnotes
Transparency Declaration
All the authors declare that they have no conflicts of interest.
References
- 1.Varon E, Mainardi JL, Gutmann L. Streptococcus pneumoniae: still a major pathogen. Clin Microbiol Infect. 2010;16:401. doi: 10.1111/j.1469-0691.2010.03190.x. [DOI] [PubMed] [Google Scholar]
- 2.Center for Disease Control Prevention. Defining the public health impact of the drug resistant Streptococcus pneumoniae: report of a working group. MMWR Morb Mortal Wkly Rep. 1996;45:1–21. [PubMed] [Google Scholar]
- 3.Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–2051. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 4.Siemieniuk R, Gregson DB, Gill MJ. The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study. BMC Infect Dis. 2011;11:314. doi: 10.1186/1471-2334-11-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasi F, Mantero M, Santus P, Tarsia P. Understanding the burden of pneumococcal disease in adults. Clin Microbiol Infect. 2012;18(suppl 5):7–14. doi: 10.1111/j.1469-0691.2012.03937.x. [DOI] [PubMed] [Google Scholar]
- 6.Herrero FS, Perez TL, Olivas JB. Bacteremic Streptococcus pneumoniae in community-acquired pneumonia: an update. Curr Respir Med Rev. 2010;6:188–193. [Google Scholar]
- 7.File TM. Community-acquired pneumonia. Lancet. 2003;362:1991–2001. doi: 10.1016/S0140-6736(03)15021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer TT, Welte T, Ernen C, et al. Cost analyses of community acquired pneumonia from the hospital perspective. Chest. 2005;128:2238–2246. doi: 10.1378/chest.128.4.2238. [DOI] [PubMed] [Google Scholar]
- 9.Marrie TJ, Durant H, Yates L. Community-acquired pneumonia requiring hospitalization: 5-year prospective study. Rev Infect Dis. 1989;11:586–599. doi: 10.1093/clinids/11.4.586. [DOI] [PubMed] [Google Scholar]
- 10.Benenson RS, Kepner AM, Pyle DN, et al. Selective use of blood cultures in emergency department pneumonia patients. J Emerg Med. 2007;33:1–8. doi: 10.1016/j.jemermed.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Afshar N, Tabas J, Afshar K, et al. Blood cultures for community- acquired pneumonia: are they worthy of two quality measures? A systematic review. J Hosp Med. 2009;4:112–123. doi: 10.1002/jhm.382. [DOI] [PubMed] [Google Scholar]
- 12.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 13.Hachel M, Lascols C, Bouchillon S, et al. Serotype prevalence and antibiotic resistance in Streptococcus pneumoniae clinical isolates among global population. Vaccine. 2013;31:4881–4887. doi: 10.1016/j.vaccine.2013.07.054. [DOI] [PubMed] [Google Scholar]
- 14.Hausdorff WP, Bryant J, Kloek C, et al. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin Infect Dis. 2000;30:122–140. doi: 10.1086/313609. [DOI] [PubMed] [Google Scholar]
- 15.Tan TQ, Mason EO, Jr, Wald ER, et al. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics. 2002;110:1–6. doi: 10.1542/peds.110.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Fedson DS, Nicolas-Spony L, Klemets P, et al. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev Vaccines. 2011;10:1143–1167. doi: 10.1586/erv.11.99. [DOI] [PubMed] [Google Scholar]
- 17.Lynch JP, Zhanel GG. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med. 2009;30:189–209. doi: 10.1055/s-0029-1202938. [DOI] [PubMed] [Google Scholar]
- 18.Miller E, Andrews NJ, Waight PA, et al. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–768. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 19.Horacio AN, Diamantino-Miranda J, Aguilar SI, et al. Serotype changes in adult invasive pneumococcal infections in Portugal did not reduce the high fraction of potentially vaccine preventable infections. Vaccine. 2012;30:218–224. doi: 10.1016/j.vaccine.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Maraki S, Mantadakis E, Samonis G. Serotype distribution and antimicrobial resistance of adult Streptococcus pneumoniae clinical isolates over the period 2001–2008 in Crete, Greece. Chemotherapy. 2010;56:325–332. doi: 10.1159/000320152. [DOI] [PubMed] [Google Scholar]
- 21.Lujan M, Gallengo M, Belmonte Y, et al. Influence of pneumococcal serotype group on outcome in adults with bacteraemic pneumonia. Eur Respir J. 2010;36:1073–1079. doi: 10.1183/09031936.00176309. [DOI] [PubMed] [Google Scholar]
- 22.Grall N, Hurmic O, Al Nakib M, et al. Epidemiology of Streptococcus pneumoniae in France before introduction of the PCV-13 vaccine. Eur J Clin Microbiol Infect Dis. 2011;30:1511–1519. doi: 10.1007/s10096-011-1251-9. [DOI] [PubMed] [Google Scholar]
- 23.Imohl M, Reinert RR, Mutscher C, et al. Macrolide susceptibility and serotype specific macrolide resistance of invasive isolates of Streptococcus pneumoniae in Germany from 1992 to 2008. BMC Microbiol. 2010;10:299. doi: 10.1186/1471-2180-10-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lismond A, Carbonnelle S, Verhaegen J, et al. Antimicrobial susceptibility of Streptococcus pneumoniae isolates from vaccinated and non-vaccinated patients with a clinically confirmed diagnosis of community-acquired pneumonia in Belgium. Int J Antimicrob Agents. 2012;39:206–216. doi: 10.1016/j.ijantimicag.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Jansen AG, Rodenburg GD, van der Ende A, et al. Invasive pneumococcal disease among adults: associations among serotypes, disease characteristics, and outcome. Clin Infect Dis. 2009;49:23–29. doi: 10.1086/600045. [DOI] [PubMed] [Google Scholar]
- 26.Johnson HL, Deloria-Knoll M, Levine OS, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7:e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musher DM, Watson DA, Dominguez EA. Pneumococcal vaccination: work to date and future prospects. Am J Med Sci. 1990;300:45–52. doi: 10.1097/00000441-199007000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Biagini RE, Schlottmann SA, Sammons DL, et al. Method for simultaneous measurement of antibodies to 23 pneumococcal capsular polysaccharides. Clin Diagn Lab Immunol. 2003;10:744–750. doi: 10.1128/CDLI.10.5.744-750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson LA, Janoff EN. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clin Infect Dis. 2008;47:1328–1338. doi: 10.1086/592691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overturf GD American Academy of Pediatrics. Committee on Infectious Diseases. Technical report: prevention of pneumococcal infections, including the use of pneumococcal conjugate and polysaccharide vaccines and antibiotic prophylaxis. Pediatrics. 2000;106:367–376. doi: 10.1542/peds.106.2.367. [DOI] [PubMed] [Google Scholar]
- 31.Pollard AJ, Perret KP, Beverley PC. Maintaining protection against invasive bacteria with protein–polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9:213–220. doi: 10.1038/nri2494. [DOI] [PubMed] [Google Scholar]
- 32.Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422. doi: 10.1002/14651858.CD000422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. Weekly epidemiological record. 2008 [Google Scholar]
- 34.Ada G. Vaccines and vaccination. N Engl J Med. 2001;345:1042–1053. doi: 10.1056/NEJMra011223. [DOI] [PubMed] [Google Scholar]
- 35.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein–polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 36.CDC. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction eight states, 1998–2005. MMWR Morb Mortal Wkly Rep. 2008;57:144–148. [PubMed] [Google Scholar]
- 37.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007;196:1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 38.Miller E, Andrews NJ, Waight PA, et al. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;10:760. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 39.French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med. 2010;362:812–822. doi: 10.1056/NEJMoa0903029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Roux A, Schm€ole-Thoma B, Siber GR, et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis. 2008;46:1015–1023. doi: 10.1086/529142. [DOI] [PubMed] [Google Scholar]
- 41.Prymula R, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367:740–748. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease C and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2012;61:816–819. [PubMed] [Google Scholar]
- 43.Regev-Yochaya G, Rahava G, Strahilevitz J, et al. A nationwide surveillance of invasive pneumococcal disease in adults in Israel before an expected effect of PCV7. Vaccine. 2013;31:2387–2394. doi: 10.1016/j.vaccine.2013.02.059. [DOI] [PubMed] [Google Scholar]
- 44.Smith KJ, Wateska AR, Nowalk MP, et al. Modeling of cost effectiveness of pneumococcal conjugate vaccination strategies in U.S. older adults. Am J Prev Med. 2013;44:373–381. doi: 10.1016/j.amepre.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pradas R, Gil de Miguel A, _Alvaro A, et al. Budget impact analysis of a pneumococcal vaccination programme in the 65-year-old Spanish cohort using a dynamic model. BMC Infect Dis. 2013;13:175. doi: 10.1186/1471-2334-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klok RM, Lindkvist RM, Ekelund M, et al. Cost-effectiveness of a 10- versus 13-valent pneumococcal conjugate vaccine in Denmark and Sweden. Clin Ther. 2013;35:119–134. doi: 10.1016/j.clinthera.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Hak E, Grobbee DE, Sanders EA, et al. Rationale and design of CAPITA: a RCT of 13-valent conjugated pneumococcal vaccine efficacy among older adults. Neth J Med. 2008;66:378–383. [PubMed] [Google Scholar]
- 48.Jackson LA, Neuzil KM, Yu O. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–1755. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 49.Jackson LA, Gurtman A, van Cleeff M, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine. 2013;31:3577–3584. doi: 10.1016/j.vaccine.2013.04.085. [DOI] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. MMWR Morb Mortal Wkly Rep. 2012;61:394–395. [PubMed] [Google Scholar]
- 51.Jackson LA, Gurtman A, Rice K, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31:3594–3602. doi: 10.1016/j.vaccine.2013.04.084. [DOI] [PubMed] [Google Scholar]
- 52.Munier AL, de Lastours V, Varon E, et al. Invasive pneumococcal disease in HIV-infected adults in France from 2000 to 2011: antimicrobial susceptibility and implication of serotypes for vaccination. Infection. 2013;41:663–668. doi: 10.1007/s15010-013-0419-x. [DOI] [PubMed] [Google Scholar]
- 53.Smith KJ, Nowalk MP, Raymund M, et al. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31:3950–3956. doi: 10.1016/j.vaccine.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]