Abstract
Mucormycosis is a life threatening condition caused by invasion of fungi of the order Mucorales. Gastrointestinal invasion is very rare and often lethal, particularly in disseminated mucormycosis. We present the case of a 26-year-old woman from North Africa with type 2 diabetes who, after a cholecystectomy, developed unexplained septic shock and haematemesis due to gastric necrosis. Computed tomography (CT) revealed a disseminated fungal invasion of the lungs, kidney and paranasal sinuses. A gastrectomy and subsequent amphotericin B treatment resolved her condition. The number of patients with mucormycosis is increasing. Early diagnosis of high risk patients with CT and biopsies from which fungi are directly isolated must be followed by surgery and systemic amphotericin B infusion.
Keywords: Gastrectomy, Mucormycosis, Sepsis
Mucormycosis is a life threatening infection caused by fungi of the subphylum Mucoromycotina, order Mucorales. Mucoromycotina are characterised by large, ribbon-like hyphae with only occasional septa (aseptate fungi). These fungi cause mucormycosis, which leads to severe and potentially life threatening infections. Patients at risk of invasive mucormycosis include those with type 2 diabetes or autoimmune disorders, solid organ and hematopoietic stem cell transplant recipients, neonates, users of illicit intravenous drugs and patients with burns, trauma or surgical wounds.1 The symptoms of mucormycosis are varied and depend on the site affected; however, no specific blood test is available.
Case History
In the summer of 2010, a 26-year-old woman of North African ethnicity was admitted to our emergency department for abdominal pain, which had developed three days previously, and fever. She complained of upper right quadrant pain with a positive Murphy’s sign, tachypnoea and tachycardia. Her blood tests showed increased leucocytes, C-reactive protein and transaminases. Her past medical history revealed type 2 diabetes mellitus. Abdominal ultrasonography identified distended, abnormal gallbladder with lithiasis. She underwent a laparoscopic cholecystectomy for suspected acute cholecystitis.
In the early postoperative period, she developed rapid and progressive respiratory failure, and was admitted to the intensive care unit with a diagnosis of severe sepsis. She deteriorated rapidly, developing multiple organ failure with kidney, lung and cardiovascular involvement despite treatment. She required vasoactive support with norepinephrine and dopamine. Blood samples from central and peripheral lines were obtained for culture, and urine was collected for analysis. She was treated with broad spectrum antibiotics and antifungal therapy.
On the third postoperative day, her blood results were: white blood cells 33,000/mm3, haemoglobin 8g/dl, haematocrit 27.1%, platelets 95,000/mm3, creatinine 9.6mg/dl, glucose 151mg/dl, pH 7.22, C-reactive protein 34mg/l and procalcitonin 15ng/ml. She underwent continuous venovenous haemofiltration. On the same day, she had an episode of haematemesis and an oesophagogastroduodenoscopy revealed gastric necrosis. This was confirmed by abdominal computed tomography (CT), which showed full-thickness oedema of the stomach with poor enhanced contrast (Fig 1), along with lung, kidney and paranasal sinus involvement (Figs 2–4). A total gastrectomy with an oesophagojejunostomy was performed. The histology was positive for the presence of fungi and revealed full-thickness necrosis of the stomach layers. Further examination of tissue specimen cultures by haematoxylin and eosin staining confirmed the diagnosis of mucormycosis. She was treated with amphotericin B and was discharged in good health three weeks later.
Figure 1.
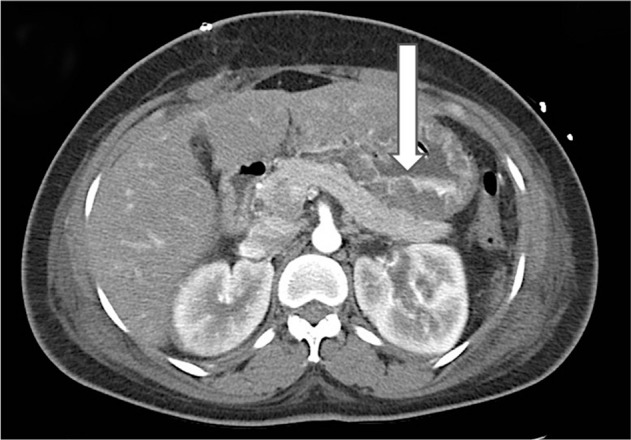
Axial computed tomography of the stomach. Arrow indicates full-thickness oedema with poor enhanced contrast due to mucormycosis invasion.
Figure 2.
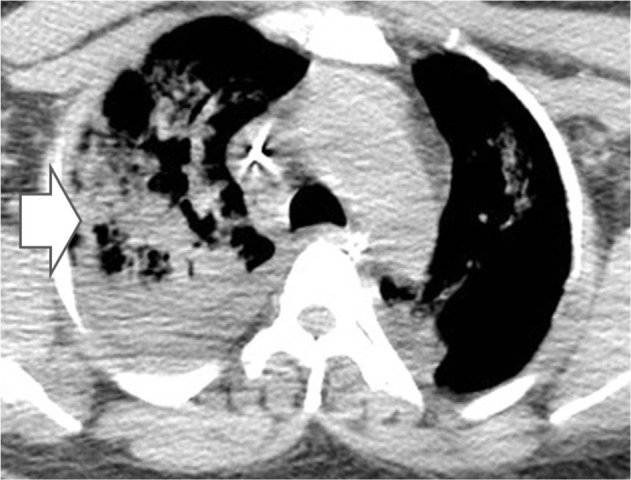
Axial computed tomography of the lung. Arrow indicates presence of lung consolidation due to mucormycosis invasion.
Figure 4.
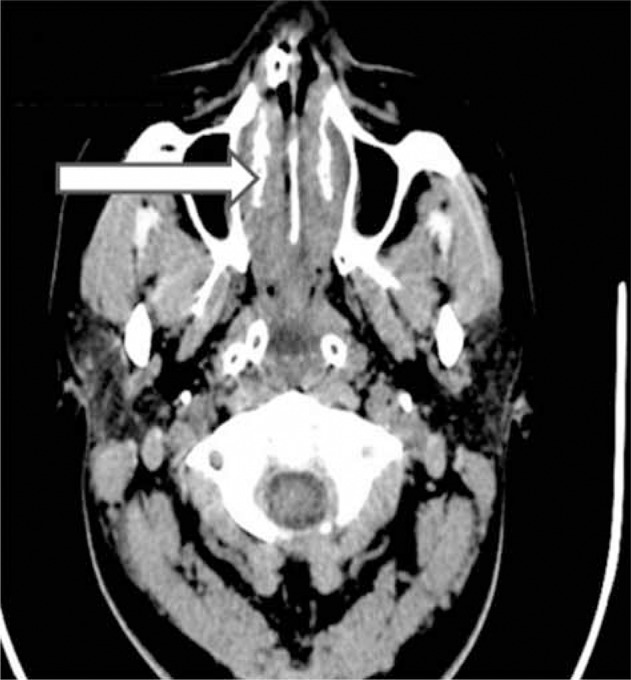
Axial computed tomography of the paranasal sinuses. Arrow indicates presence of infection due to mucormycosis invasion.
Figure 3.
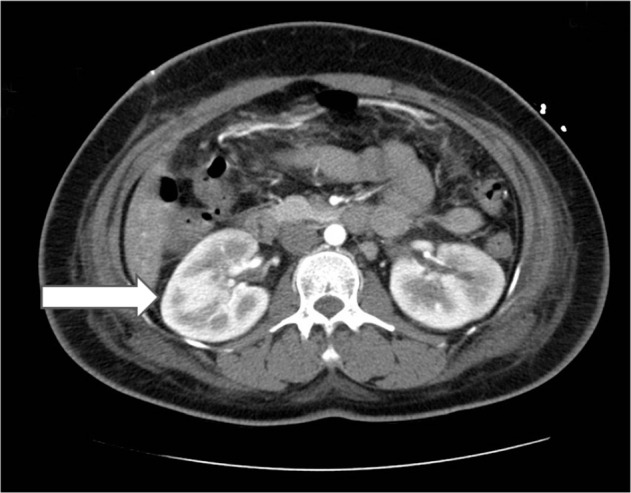
Axial computed tomography of the kidney. Arrow indicates presence of kidney infection due to mucormycosis invasion.
Discussion
Disseminated mucormycosis has been reported in patients with cerebral (48% of cases), cutaneous (39%) and pulmonary (20%) disease but not in gastrointestinal disease.2 Non-specific abdominal pain and distension associated with nausea and vomiting are the most common symptoms; however, fever, haematochezia and haematemesis may also occur.1 The non-specific presentation of symptoms often leads to late diagnosis. Despite aggressive antifungal and surgical management, the mortality rate of gastric mucormycosis remains high.3
The pathogenesis of mucormycosis is unclear although the fungus thrives in an acidic environment and the mode of transmission is typically airborne, which commonly leads to sinus and pulmonary manifestations. Neutrophils and macrophages are the primary host defence against these organisms, which can be impaired by acidosis and hyperglycaemia. Phagocyte dysfunction combined with increased availability of iron explains why patients with neutropaenia and diabetic ketoacidosis are at an increased risk of this infection.3
Diagnostic options for mucormycosis remain limited to clinical and radiographic findings, together with staining and culture. Although these methods are useful, they are limited in terms of sensitivity, time to detection and ability to provide rapid results. No serological or polymerase chain reaction-based tests for rapid diagnosis of mucormycosis are available and up to half of cases are diagnosed post mortem, underscoring the critical need to maintain a high index of clinical suspicion and to aggressively pursue diagnostic biopsy.2 Cultures grown in most routine laboratory media will show fluffy white, gray or brownish colonies, which fill the container in a few days. They are commonly found in soil and decaying organic matter including food items such as fruits, bread and seeds.1
The consensus in the literature is that several factors are critical to eradicating mucormycosis. These include rapidity of diagnosis, reversal of the underlying predisposing factors, appropriate antifungal therapy and, most importantly, appropriate surgical debridement of infected tissue. Angioinvasion, thrombosis and tissue necrosis associated with mucormycosis lead to poor penetration of antifungal agents at the sites of infection and compromise their efficacy. Consequently, debridement of necrotic tissues plays a crucial role in the treatment of mucormycosis. As reported by Spellberg, patients who did not undergo surgical debridement of mucormycosis had a far higher mortality rate than patients who underwent surgery.4
It is important for the radiologist to understand the importance of identifying other sites of infection on imaging. CT can be instrumental in identifying multiorgan involvement.5 The signs of pulmonary mucormycosis on chest imaging are also non-specific and indistinguishable from those of pulmonary aspergillosis. The most frequent findings include infiltration, consolidation, nodules, cavitations, atelectasis, effusion, posterior tracheal band thickening, hilar or mediastinal lymphadenopathy and even normal findings. Rhinocerebral CT shows oedematous mucosa, fluid filling the ethmoid sinuses, and destruction of periorbital tissues and bone margins.
Data from several countries indicate that the incidence of mucormycosis is increasing in patients with haematological disorders, malignancies or diabetes mellitus and in transplant recipients. Increasing numbers of immunocompromised hosts, widespread use of antifungal agents inactive against mucormycosis or other unidentified factors may be contributing to this situation.1
Conclusions
We have described the case of an immunocompromised patient who developed unexplained and early onset, rapidly evolving sepsis. She was treated with a broad spectrum antibiotic and antifungal (amphotericin B), and owing to gastric necrosis, a total gastrectomy was performed. She underwent CT for diagnosis of mucormycosis. Oesophagogastroduodenoscopy can be helpful if the patient develops haematemesis or haematochezia. Surgical removal of the infected site is essential to increase the survival rate of mucormycosis, which remains quite poor.
References
- 1.Walsh TJ, Gamaletsou MN, McGinnis MR et al Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis 2012; 54: S55–S60. [DOI] [PubMed] [Google Scholar]
- 2.Sun HY, Singh N. Mucormycosis: its contemporary face and management strategies. Lancet Infect Dis 2011; 11: 301–311. [DOI] [PubMed] [Google Scholar]
- 3.Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev 2005; 18: 556–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spellberg B. Gastrointestinal mucormycosis: an evolving disease. Gastroenterol Hepatol 2012; 2: 140–142. [PMC free article] [PubMed] [Google Scholar]
- 5.Samet JD, Horton KM, Fishman EK. Invasive gastric mucormycosis: CT findings. Emerg Radiol 2008; 15: 349–351. [DOI] [PubMed] [Google Scholar]


