Figure 10.
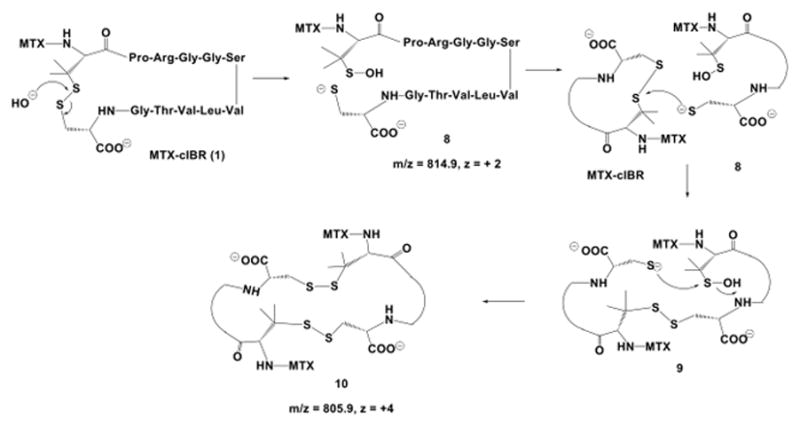
The disulfide bond degradation in MTX-cIBR at pH 12 produced thiolate-sulfenic acid 8 and cyclic dimer 10. The disulfide bond opening was via direct attack of hydroxyl anion on the sulfur of the disulfide bond. Thiolate 8 was reacted with another molecule of the conjugate to produce a linear dimer 9; intramolecular reaction of thiolate anion with the sulfur atom of sulfenic acid generated a cyclic dimer 10.
