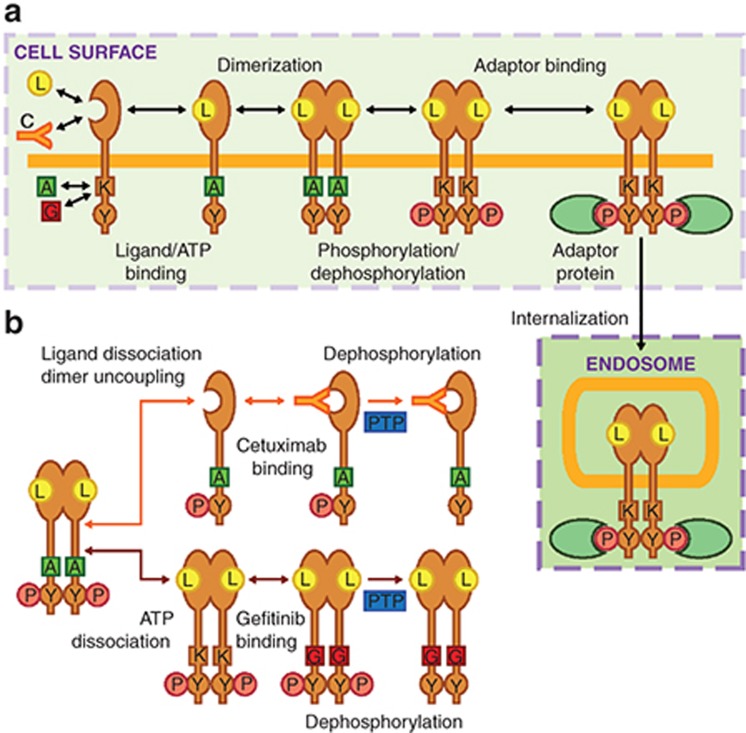
Figure 1.
Model topology of processes leading to epidermal growth factor receptor (EGFR) phosphorylation and therapeutic inhibition of this process. (a) Ligand (L) and cetuximab (C) compete for binding to the EGFR extracellular domain while ATP (A) and gefitinib (G) compete for binding to the EGFR kinase domain (K). Ligand binding promotes EGFR dimerization and ATP-dependent phosphorylation (P) of cytoplasmic tyrosines (Y), enabling receptor binding to intracellular adaptor proteins (e.g., GRB2). EGFR-GRB2 binding permits EGFR internalization. (b) Gefitinib and cetuximab mediate reductions in the phosphorylation of dimerized and phosphorylated EGFR through different mechanisms. ATP dissociation from EGFR allows gefitinib to bind, prolonging the time the receptor remains dephosphorylated after being acted upon by protein tyrosine phosphatases (PTP). Cetuximab can bind phosphorylated receptors once they uncouple from dimers and ligands dissociate, which slows further rounds of ligand binding and dimerization and prolongs the time the receptor remains dephosphorylated after being acted upon by PTPs.