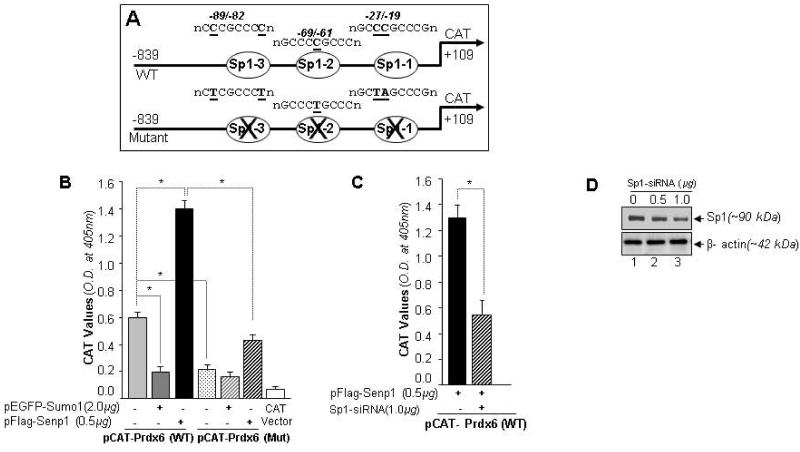
Fig. 11.
Sumoylation and deSumoylation of Sp1 was involved in regulating Prdx6 transcription. (A) Schematic illustration of wild-type Prdx6 gene promoter construct (−839/+109) containing three Sp1-binding elements (WT) and its mutant (all three Sp1 sites disrupted using site-directed mutagenesis) linked to CAT vector. (B) Prdx6 promoter activity was down regulated in cells overexpressing Sumo1 compared to cells overexpressing Senp1. hLECs were co-transfected with wild-type Prdx6 promoter linked to CAT vector or mutated construct (at all three Sp1 sites) along with either pEGFP-Sumo1 or pFlag-Senp1. The effects of pEGFP-Sumo1 (dark gray bar) or pFlag-Senp1 (black bar) on CAT activity are shown. Empty CAT-vector served as control (open bar). We did not observe significant changes in pCAT-Prdx6 mutant coexpressed with either pEGFP-Sumo1 or pFlag-Senp1, suggesting that Prdx6 transcription was linked to Sumoylation/deSumoylation of Sp1. The transfection efficiencies were normalized using cotransfected pEGFP-vector. Data represent the mean ± SD from three independent experiments (*, p<0.001). (C) Sp1-siRNA assay confirming the involvement of Sumo1 and Senp1 in modulating Prdx6 transcriptional activity through Sp1. hLECs were transiently cotransfected with pCAT-Prdx6 reporter plasmid and pFlag-Senp1 with or without siRNA specific to Sp1, as indicated. CAT activity was monitored. The results are represented as a histogram showing that promoter activity was significantly reduced in Sp1-siRNA transfected cells compared to untransfected cells (lined bar vs black bar). Transfection efficiencies were normalized using pEGFP vector. Data represent the mean ± SD from three independent experiments (*, p<0.001). (D) Silencing of Sp1 by specific siRNA was validated by Western blotting. The membrane was striped and stained with β-actin antibody.