FIG 1.
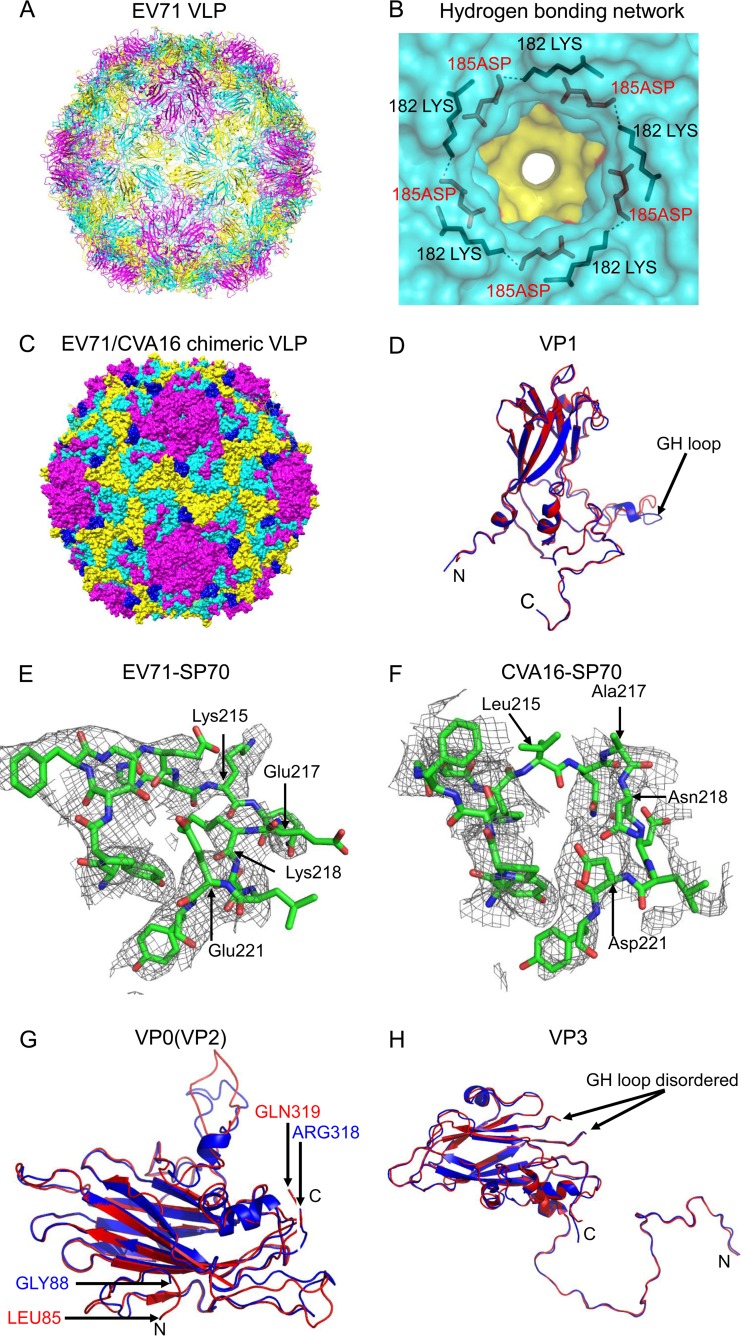
Crystal structures of EV71 VLP and EV71/CVA16 chimeric VLP. (A) Cartoon representation of EV71 VLP viewed along the 2-fold axis with VP1, VP0, and VP3 colored in magenta, yellow, and cyan, respectively. (B) Hydrogen-bonding network at the 5-fold axis channel. The amino group in the side chain of Lys182 (colored in black) of VP1 interacted with Asp185 (colored in red) of a neighboring VP1 through hydrogen bonds. (C) Surface representation of EV71/CVA16 chimeric VLP viewed along the 2-fold axis. The capsid proteins VP1, VP0, and VP3 are colored in magenta, yellow, and cyan, respectively. The CVA16-SP70 epitope is colored in blue. (D) Superimposition of VP1 from EV71 VLP and EV71/CVA16 chimeric VLP. Residues 72 to 297 in EV71 VLP (colored in blue) and residues 72 to 296 in EV71/CVA16 chimeric VLP (colored in red) are modeled. (E) Electron densities corresponding to the EV71-SP70 epitope (amino acids 208 to 222 in VP1) in EV71 VLP. These residues are displayed as sticks with Lys215, Glu217, Lys218, and Glu221 labeled. (F) Electron densities corresponding to the CVA16-SP70 region (amino acids 208 to 222 in VP1) in the chimeric VLP. These residues are displayed as sticks with Leu215, Ala217, Asn218, and Asp221 labeled. (G) Superimposition of VP0 from EV71 VLP and EV71/CVA16 chimeric VLP. The color scheme is the same as in panel D. Residues 88 to 318 in EV71 VLP and residues 85 to 319 in EV71/CVA16 chimeric VLP are modeled. (H) Superimposition of VP3 from EV71 VLP and EV71/CVA16 chimeric VLP. The color scheme is the same as in panel D. Residues 1 to 176 and 190 to 237 in EV71 VLP and residues 1 to 176 and 189 to 236 in EV71/CVA16 chimeric VLP are modeled.