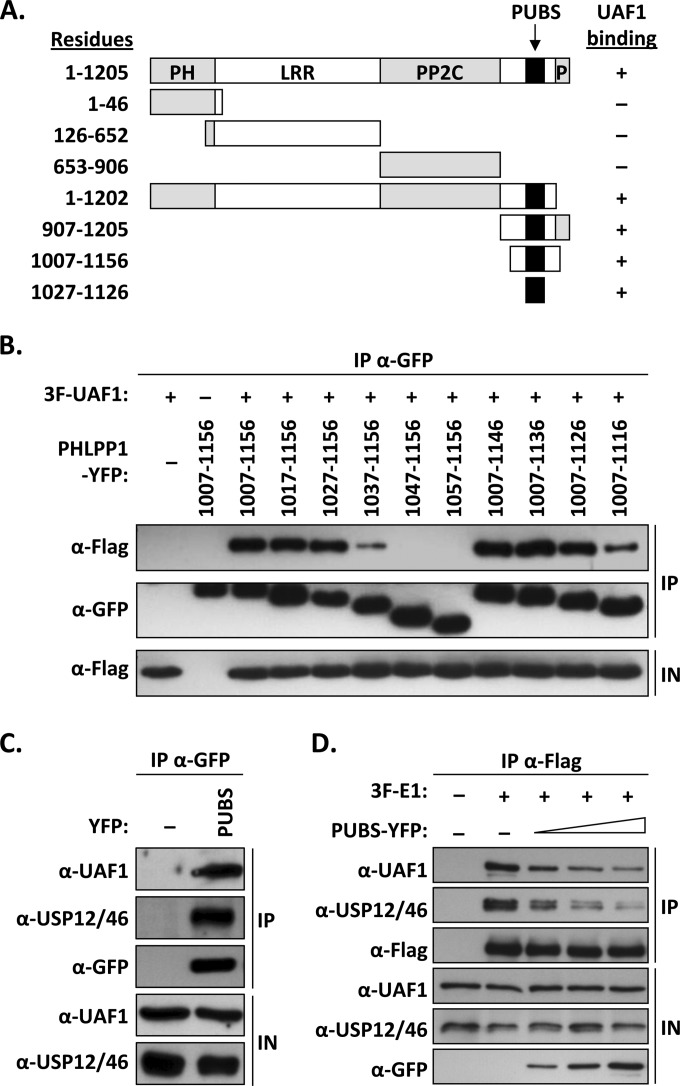
FIG 2.
Mapping of the UAF1-binding site of PHLPP1. (A) Schematic representation of PHLPP1 and truncated derivatives. Functional regions of the protein are indicated: pleckstrin homology domain (PH), leucine-rich region (LRR), PP2C phosphatase domain (PP2C), and C-terminal PDZ protein-binding domain (P). The position of the PHLPP1 UBS (PUBS; amino acids 1027 to 1126) is indicated by a black box. Amino acid boundaries are indicated on the left. Results from coimmunoprecipitation experiments are summarized on the right (+, binding; −, no binding). (B) Fine mapping of PUBS. C33A cells were cotransfected with an expression vector for 3F-UAF1 together with a plasmid encoding one of the indicated PHLPP1 fragments fused to YFP. YFP-PHLPP1 proteins were immunoprecipitated with anti-GFP antibodies and the presence of 3F-UAF1 in the immunoprecipitates (IP) and input cell extracts (IN) tested by Western blotting using an anti-Flag antibody. (C) Coimmunoprecipitation of endogenous UAF1 and USP12/46 with PUBS. C33A cells were transfected with a plasmid encoding PUBS-YFP or YFP alone (−) as a negative control. YFP proteins were immunoprecipitated as described above, and the immunoprecipitates were probed for the presence of UAF1 and USP12/46 by Western blotting using antibodies against these proteins. (D) Inhibitory effect of PUBS-YFP on the interaction of 3F-E1 with endogenous UAF1 and USP12/46. C33A cells were transfected with 4 μg of 3F-E1 expression vector and increasing amounts of PUBS-YFP expression plasmid (1, 2, and 4 μg). 3F-E1 was immunoprecipitated with an anti-Flag antibody and the presence of coprecipitated UAF1 and USP12/46 analyzed by Western blotting using antibodies against these proteins.