Figure 4.
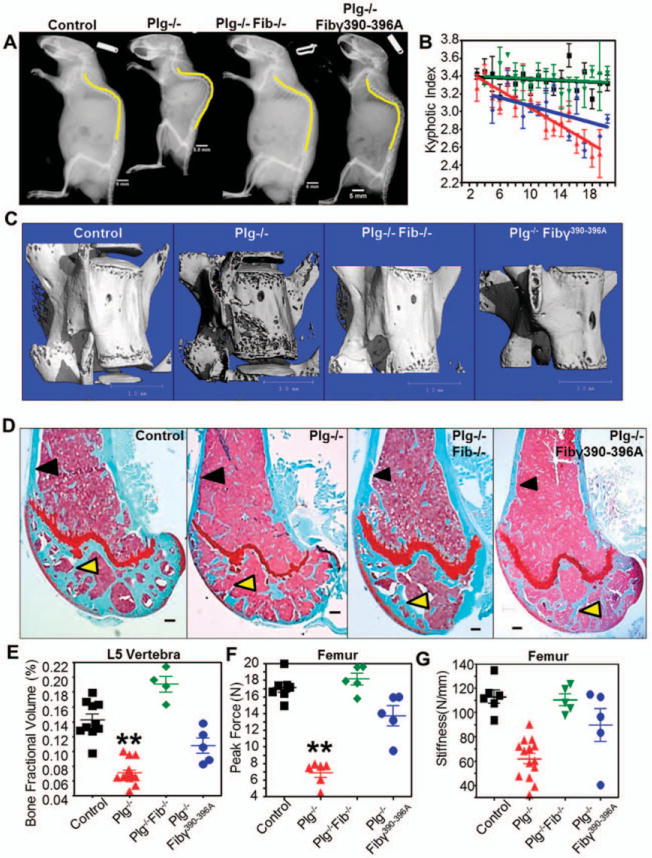
Targeting fibrin prevents bone loss in plasminogen-deficient (PLG−/−) mice. A, Lateral radiographs at age 20 weeks demonstrate that the degree of kyphosis seen in PLG−/− mice is reduced in mice deficient in both plasminogen and fibrinogen (PLG−/−FBG−/− mice [or Plg−/−Fib−/− mice]) as well as in plasminogen-deficient mice expressing a mutant fibrinogen unable to bind αMβ2 (PLG−/−FBGg390–396A mice [or Plg−/−Fib7390–396A mice]). Bars = 5 mm. B, By age 20 weeks there is complete prevention of kyphosis in PLG−/−FBG−/− mice (green symbols) and partial prevention in PLG−/−FBGg390–396A mice (blue symbols). Wild-type (WT) mice are represented by black symbols; PLG−/− mice are represented by red symbols. Bars show the mean ± SD. C, Three-dimensional reconstruction of micro-computed tomography data from L5 vertebrae in 20-week-old mice shows decreased bone loss in PLG−/−FBG−/− and PLG−/−FBGg390–396A mice compared to PLG−/− mice. Bars = 1 mm. D, Similar reductions in cortical bone loss (black arrows) and trabecular bone loss (yellow arrows) are observed in Safranin O-stained sections of distal femurs from 20-week-old PLG−/−FBG−/− and PLG−/−FBGg390–396A mice. Bars = 200 /μm. E-G, These changes in bone architecture are reflected by enhanced bone fraction volume (E) and biomechanical properties contributing to fracture resistance (peak force [F] and stiffness [G]). Symbols represent individual mice (n = 10 WT mice; n = 8 PLG−/− mice; n = 5 PLG−/−FBG−/− mice; n = 5 PLG−/−FBGg390–396A mice). Bars show the mean ± SD. ** = P < 0.01 versus control, by one-way analysis of variance. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.38639/abstract.
