Abstract
In the present study we have investigated the population genetic structure of albacore (Thunnus alalunga, Bonnaterre 1788) and assessed the loss of genetic diversity, likely due to overfishing, of albacore population in the North Atlantic Ocean. For this purpose, 1,331 individuals from 26 worldwide locations were analyzed by genotyping 75 novel nuclear SNPs. Our results indicated the existence of four genetically homogeneous populations delimited within the Mediterranean Sea, the Atlantic Ocean, the Indian Ocean and the Pacific Ocean. Current definition of stocks allows the sustainable management of albacore since no stock includes more than one genetic entity. In addition, short- and long-term effective population sizes were estimated for the North Atlantic Ocean albacore population, and results showed no historical decline for this population. Therefore, the genetic diversity and, consequently, the adaptive potential of this population have not been significantly affected by overfishing.
Introduction
Albacore tuna (Thunnus alalunga, Bonnaterre 1788) is distributed in the Atlantic, Pacific and Indian Oceans and in the Mediterranean Sea, extending from 50–55°N to 40–45°S [1]. This species is the fourth most important one of the Thunnus genus with regard to captures [2]. This fact reflects the high commercial value of the albacore and its related products, which makes this species likely to be exploited beyond its maximum sustainable yield [1]. Migrations on this species has been studied for several decades through tag-recapture experiments showing low rate of albacore migration between hemispheres [3], and no transoceanic [3,4,5] neither Atlantic-Mediterranean migrations [6]. There are very few studies on spawning areas of this species, because catching larvae or young-of-the-year individuals (reference samples) of this species is not a very common event. One spawning ground has been defined in the western Mediterranean [7–9], two spawning areas in the North Atlantic Ocean [10,11], a single one in the South Atlantic [10], one spawning area in the Indian Ocean [12,13], and two Pacific separate spawning grounds: north and south [14–16]. According to this knowledge on population dynamics of albacore, six stocks or management units are currently defined by Regional Fisheries Management Organizations (RFMOs): (i) Mediterranean Sea, (ii) North Atlantic Ocean, (iii) South Atlantic Ocean, (iv) Indian Ocean, (v) North Pacific Ocean and (vi) South Pacific Ocean. Many fisheries are regulated in accordance with spatial schemes. However, management units based only on knowledge about migrations do not necessarily correspond to the biological structure of the populations [17,18]. In these cases, when fishery management is not based or does not fit the biological structure, changes may occur in the biological attributes, productivity and genetic diversity of the exploited species [19]. Therefore, the establishment of an accurate population boundary for a commercial species requires a multidisciplinary approach, and genetic studies can contribute very valuable information in this regard [20,21]. Thus, studies including population genetic structure assessment together with other population identification methodologies, such as tag-recapture [6] or chemical tags in otoliths [22], have become more common in the last decade. These multidisciplinary studies allow a more accurate population structure and hence, more sustainable fisheries management policies.
A variety of studies have assessed population structure of albacore species using multiple approaches including: otolith microstructure [23,24], tag-recapture methods [6], morphometrics [25] and genetic techniques [26–34]. The population structure of albacore has been found to exhibit a high dispersal capacity (e.g. [35]), similarly to what happens to other marine species such as Atlantic bluefin tuna (Thunnus thynnus) [34,36] or Atlantic mackerel (Scomber scombrus) [37]. However, despite the number of studies performed since the last decade, genetic structure of albacore is not clear yet, since contradictory information about number of albacore populations and population boundaries have been reported. In this regard, Albaina et al. [34] suggested four albacore populations (one in each ocean and one in the Mediterranean Sea), but Pujolar et al. [38] and Graves and Dizon [27] found genetic homogeneity between the Atlantic Ocean and Mediterranean Sea and Atlantic and Pacific Oceans, respectively, and Montes et al. [33] found homogeneity between the Atlantic and Indian Oceans. Moreover, genetic structure within oceans remains unclear since heterogeneity within them or within the Mediterranean Sea has been suggested [6,26,29,32,33,39]. Comparison between studies is difficult because differences on genetic markers studied and also on geographic areas assessed, which in certain studies are very limited into the bargain. In fact, few studies have addressed the population structure of albacore covering the worldwide distribution range of the species [1,33,34].
The North Atlantic albacore tuna stock was subjected to overfishing conditions between the mid 1960s and mid 2000s. As a result, the spawning stock biomass had been overexploited (below levels associated to the maximum sustainable yield) since the 1980s, but is now recovering over the last decade [40].After the population genetic structure of a species is defined, an essential parameter that informs about the sustainable management and conservation of exploited species is the effective population size (Ne) [41]. While population genetic structure enables a definition of populations, that can be linked to the stock or management unit concept, Ne determines how vulnerable these populations are to losing genetic diversity due to genetic drift [42] and consequently, this variable assesses their responsiveness and adaptation capabilities. Despite the importance of this parameter for populations’ conservation, few studies have estimated Ne for tunas [36,43,44,45,46].
In summary, a number of outstanding issues persist which have direct implications for the sustainable management of albacore. These main questions to be answered include (1) the absence of a consensus about the genetic structure of this species worldwide, and (2) uncertainty about the impact of fishing on the effective population size (Ne) and, therefore, on the genetic diversity of albacore populations. The goal of this study is to obtain a clear definition of the population genetic structure of albacore, and to shed light on its genetic viability via the estimation of Ne for the North Atlantic population, with the aim of providing a more rational foundation for sustainable fishery management. With this objective in mind, we carried out the most extensive sampling of albacore to date, covering its worldwide distribution range (1,331 samples from 26 locations worldwide). The number of markers employed was also the highest used to date, involving 115 novel nuclear SNP markers which we report in albacore tuna through cross-species transcriptome amplification and sequencing.
Material and Methods
Samples and DNA extraction
An exhaustive spatial-temporal sample of 1,331 albacore individuals from 26 locations covering the whole geographical distribution of the species was obtained (Fig 1, Table 1). The total sample includes 774 individuals from the Atlantic Ocean (12 locations sampled over 24 years), 254 individuals from Mediterranean Sea (7 locations sampled over 12 years), 136 individuals from the Indian Ocean (4 locations within 4 years of sampling), and 167 individuals from the Pacific Ocean (3 locations sampled over 5 years). Individuals were mainly sampled between 2008 and 2012, with some individuals sampled in previous years as far back as 1988. Sampled individuals were provided either by commercial or recreational vessels or by oceanographic institutes that collected the samples during scientific surveys. All fish were collected as part of authorized routine fishing procedures and therefore did not require any special additional permission. Some samples were used in previous studies [6,32–34,47] (Table 1). Collected tissues mainly consisted of muscle, fin or heart tissue, and they were stored either frozen at -20°C or preserved in 96% ethanol at 4°C. Additionally, spine cuts mounted in Eukitt (O. Kindler GmbH), as well as dried and stained blood samples were collected (Table 1). DNA from muscle, fin and heart tissue samples was extracted using NucleoSpin 96 Tissue Kit (Macherey-Nagel). Spine and blood samples were first immersed in xylol, and spine samples were afterwards manually crushed; DNA from these samples was extracted by means of a specific membrane using QIAmp DNA Investigator Kit (Qiagen). DNA from all samples was quantified using both a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA) and a Qubit 2.0 (Invitrogen, Life Technologies) fluorometer. All DNA samples were stored at -20°C for subsequent analyses.
Fig 1. Sampling locations.
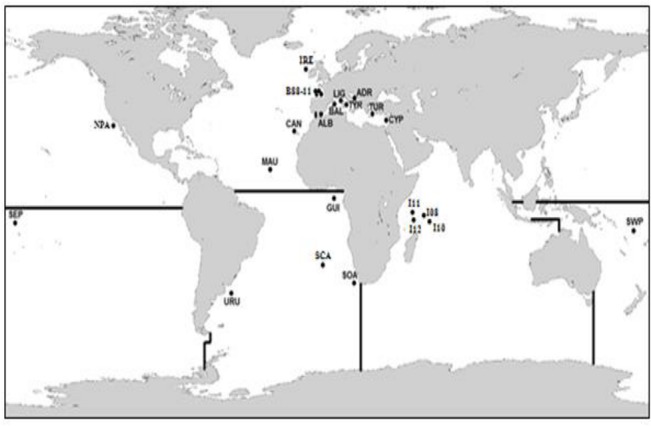
Current stock boundaries delineated with black lines. Sample abbreviations are as defined in Table 1.
Table 1. Sample number (#), name, location, year of capture, number of individuals (N), stock, geographic coordinates and sample type (T, muscle, fin or heart tissue; B, blood; S, spine).
| # | Name | Location | Year | N | Stock | Latitude | Longitude | Sample type |
|---|---|---|---|---|---|---|---|---|
| 1 A | ADR | Adriatic Sea | 2006 | 48 | Mediterranean Sea | 41.29 | 17.52 | T |
| 2 A , B , C | BAL | Balearic Sea | 2005 | 31 | Mediterranean Sea | 40.00 | 1.58 | T |
| 3 | CYP | Cyprus | 2011 | 10 | Mediterranean Sea | 36.08 | 33.68 | T |
| 4 | TUR | Turkey | 2011 | 53 | Mediterranean Sea | 35.04 | 26.80 | T |
| 5 A | TYR | Tyrrhenian Sea | 2008 | 48 | Mediterranean Sea | 38.88 | 11.74 | T |
| 6 | LIG | Ligurian Sea | 2011 | 27 | Mediterranean Sea | 43.38 | 9.05 | T |
| 7 D | ALB | Alboran Sea | 1999 | 37 | Mediterranean Sea | 36.23 | -2.00 | B |
| 8 E | B88 | Bay of Biscay | 1988 | 34 | North Atlantic Ocean | 45.10 | -4.35 | S |
| 9 E | B89 | Bay of Biscay | 1989 | 30 | North Atlantic Ocean | 45.64 | -4.76 | S |
| 10 A , B | B09 | Bay of Biscay | 2009 | 42 | North Atlantic Ocean | 45.05 | -5.28 | T |
| 11 | B10 | Bay of Biscay | 2010 | 240 | North Atlantic Ocean | 45.71 | -5.53 | T |
| 12 | B11 | Bay of Biscay | 2011 | 31 | North Atlantic Ocean | 44.92 | -4.16 | T |
| 13 | CAN | Canary Islands | 2012 | 41 | North Atlantic Ocean | 27.73 | -17.25 | T |
| 14 A , B | IRE | Ireland | 2008 | 57 | North Atlantic Ocean | 54.17 | -12.89 | T |
| 15 | MAU | Mauritania | 2010 | 48 | North Atlantic Ocean | 9.79 | -32.16 | T |
| 16 | GUI | Gulf of Guinea | 1999–2000 | 32 | South Atlantic Ocean | 1.98 | -16.58 | B |
| 17 | URU | Uruguay | 2005, 2007–2012 | 84 | South Atlantic Ocean | -36.19 | -53.16 | T |
| 18 A , B | SCA | South Africa | 2009 | 98 | South Atlantic Ocean | -24.56 | 4.42 | T |
| 19 | SOA | South Africa | 2011 | 37 | South Atlantic Ocean | -34.34 | 18.00 | T |
| 20 A , B | I08 | Seychelles | 2008–2009 | 23 | Indian Ocean | -7.11 | 54.65 | T |
| 21 | I10 | Seychelles | 2010 | 38 | Indian Ocean | -7.27 | 56.32 | T |
| 22 | I11 | Seychelles | 2011 | 42 | Indian Ocean | -7.28 | 49.06 | T |
| 23 | I12 | Seychelles | 2012 | 33 | Indian Ocean | -8.86 | 49.13 | T |
| 24 A , B | NPA | California | 2008 | 83 | North Pacific Ocean | 43.50 | -127.00 | T |
| 25 A , B | SEP | New Caledonia | 2004–2005 | 51 | South Pacific Ocean | -19.01 | -152.84 | T |
| 26 A , B | SWP | French Polynesia | 2003–2008 | 33 | South Pacific Ocean | -18.53 | 165.97 | T |
| 1988–2012 | Total = 1,331 |
SNP selection and genotyping
The SNPs used in this study were previously discovered in the closely related ABFT species through transcriptome and genome sequencing, using 454 (GS FLEX Titanium) and HiSeq2000 (Illumina), respectively (Cariani et al. Personal Communication). Of all the discovered SNPs in the ABFT species, 384 transcriptome SNPs were genotyped using the GoldenGate platform (VeraCode), in 30 albacore samples covering the entire distribution range of the species (5 individuals from each defined management unit: North Atlantic, South Atlantic, Mediterranean Sea, Indian Ocean, North Pacific and South Pacific). From the 384 ABFT SNPs, only those that successfully amplified in albacore (conversion rate) and have Minor Allele Frequency (MAF) values above 0.01 in the latter species were taken into account. From these, only those markers that were compatible with TaqMan OpenArray technology (Life Technologies) were selected for this study. Additionally, two nuclear SNPs, previously described for albacore ([34]; S1 Table), were included in the final SNP set as a positive control, in order to corroborate the correctness of the genotyping procedure. Thus, a final set of 117 SNPs was designed to genotype 1,331 albacore individuals through TaqMan OpenArray technology. Validation rate was calculated as the proportion of SNP with a MAF > 0.001. In order to ensure genotyping quality, SNPs needed to comply with the following criteria: a call rate higher than 80%, clear genotyping clusters, and compliance with Hardy-Weinberg equilibrium (HWE).
Statistical analysis
GENEPOP v4.0 [48] software was used to test departures from HWE and to analyze genotypic disequilibrium (GD) between SNPs (p-value < 0.001). Linked SNPs were phased into haplotypes using PHASE v2.1 software [49].
In order to assess the genetic population structure of the albacore, Reynolds genetic distance matrices [50] were obtained using Populations v1.2.32 software [51]. A Neighbor-Net dendrogram was constructed using SPLITSTREE v4.13 [52] based on the matrix of genetic distances. Geographic distance was calculated measuring the shortest distance by sea between each pair of sample location using scripts from the Movable Type Ltd webpage (http://www.movable-type.co.uk/scripts/latlong.html). Isolation by distance (IBD) was tested evaluating the correlation between Rousset's genetic distance [53] and geographic distance, using Mantel test implemented in IBDWS [54] with 30,000 randomizations. Population genetic structure was also assessed using STRUCTURE v2.3.4 [55] and GENELAND v3.2.2 [56] software, which are based on Bayesian clustering algorithms that allow assigning individuals to a group without previous assumption of either population units or population boundaries. STRUCTURE was run using the mixed ancestry model and correlated allele frequencies [57], using information regarding sampling location. Ten independent runs were simulated for each potential number of populations (K) with values of K = 1–6, and with a burn-in period of 50,000 Markov chain Monte Carlo (MCMC) steps, followed by 500,000 MCMC steps. The best K was estimated as proposed by Pritchard et al. [55]. CLUMPP v1.1.2 [58] was used to determine the optimal assignation of clusters for the analyzed individuals, maximizing similarity between the 10 different STRUCTURE replications for the selected K. Individual membership coefficients were graphically shaped with DISTRUCT v1.1 [59]. Finally, to test potential weaker structure within the detected major clusters, STRUCTURE analysis was repeated for each of them. While STRUCTURE is based only on the individual genotype data to infer the population structure, GENELAND uses the geographical information of the individuals as an additional parameter in the analysis. In the latter case, K was estimated from 1 to 5, using 500,000 MCMC iterations and 1,000 thinnings. Ten runs with fixed K were then post processed using a burn-in of 50,000 iterations to obtain the posterior probabilities of population membership for each individual and each pixel of the spatial domain.
We searched for candidate loci under selection (outlier loci) using the Bayesian likelihood method, as implemented in BAYESCAN v2.1 [60], with 10 pilot runs of 5,000 iterations and an additional burn-in of 50,000 iterations (sample size of 5,000 and thinning interval of 10). Critical values for the test were adjusted with false discovery rate (FDR) procedure (q-value < 0.05) [61]. Pairwise FST [62] values among samples based on neutral markers were estimated with FSTAT v2.9.3 software [63]. P-values were weighted using the FDR method for multiple testing [61].
The statistical power required to detect various levels of differentiation with the SNPs used in this study was estimated using POWSIM version 4.1 [64]. Since POWSIM is restricted to 50 loci, we selected those 50 loci with highest FST values. Burn-in consisted of 1000 steps followed by 100 batches of 1000 steps. Chi-square probabilities were used to test the significance of an FST value for each replicate run. The number of significant FST values in 1000 replicate simulations provided an estimate of the statistical power for a given level of divergence, which was controlled by allowing frequencies to drift for a given number of generations. Simulated effective populations sizes equaled 2000 fish.
Two different time-scale Ne estimates were obtained for the North Atlantic stock. Short-term Ne was estimated from temporal fluctuations in allele frequencies between cohorts [65], and a correction for overlapping generations was applied [66–68]. Generation time (Ĝ) was estimated following Felsenstein [69] from age frequency data of analyzed years (1988–2012), and changes in allele frequencies among cohorts were measured by FS [67]. The long‐term Ne [70] uses a maximum likelihood estimator based on the coalescence theory. It is a retrospective model of population genetics which traces back for the most recent individual from which all organisms in a group are directly descended, the most recent common ancestor (MRCA). This tool has been employed to estimate historic population sizes for a range of species [71].
Data for North Atlantic albacore were obtained between 1988–2012, which constitutes 4–5 generations of albacore assuming 50% maturity at age 5 [25]. Age was estimated using length and weight information according to Santiago [72] and Santiago and Arrizabalaga [47]. We used age-structure data for seven cohorts (Fig 2). Cohort analysis was carried out to assess temporal fluctuations in population size. The adult population size (Nc) in the North Atlantic, obtained from the report of the 2013 ICCAT North and South Atlantic albacore stock assessment [40], was compared with total population size Ne estimates. MIGRATE v3.2.1 software [73] was used for long‐term Ne estimation, and mutation was modeled by an infinite allele model.
Fig 2. Diagram of the defined cohorts, based on the age of the individuals.
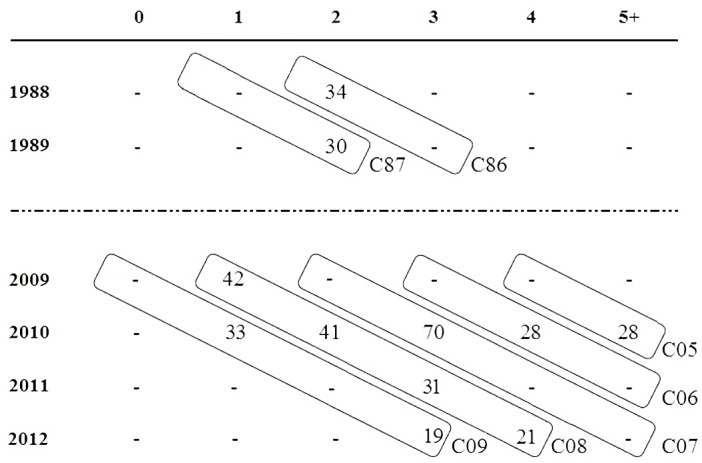
Columns indicate age of individuals and rows year of capture. Values inside tables are the number of individuals for each age/year combination. Seven cohorts were defined by diagonal frames whose names were based on the hatching year of the individuals: C86, C87, C05, C06, C07, C08 and C09.
Results
SNP selection and genotyping
From the 384 ABFT SNPs analyzed in the 30 albacore sample, 311 SNPs (conversion rate = 80.99%) successfully amplified in albacore, and among them, 121 showed MAF > 0.01 (31.51%). From these, 115 SNPs exhibited compatibility with the TaqMan OpenArray technology (Life Technologies), and were further genotyped together with the 2 nuclear SNPs included as a positive control.
Out of 117 nuclear SNPs, 95 were polymorphic (they had a MAF value above 0.001, i.e. the minor allele was observed at least 5 times) and had a clear genotype for the 1,331 albacore individuals (S1 Table). Therefore, validation rate was 24.61% (95/386). From these, 76 met HWE. The exact tests for genotypic disequilibrium (GD) detected 2 SNPs (ss974292126 and ss974292127) with significant GD probabilities, so these 2 SNPs were phased into one haplotype (ss974292126+ss974292127). Therefore, a set of 75 independent nuclear markers was downstream analyzed.
Population structure
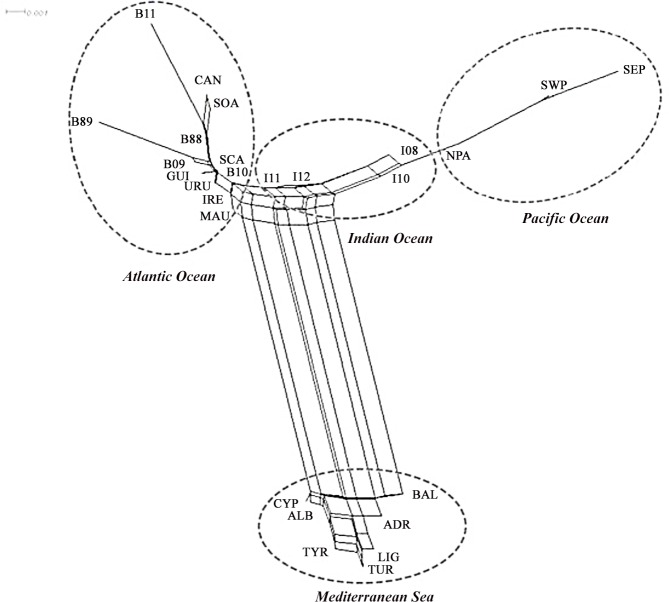
The Neighbor-Net drawn from Reynolds genetic distances (Fig 3) grouped locations according to their geographical region. The Mediterranean Sea samples, grouped into a single cluster, were the most distant from the rest. The samples from the three oceans also grouped by ocean, and those from the Indian Ocean were placed between those of the Atlantic and those of the Pacific. The genetic and geographic distances for the 26 samples showed a significant correlation (r = 0.4577, p < 0.0001; S1 Fig). This correlation increased notably when the Mediterranean samples were removed from the analysis (r = 0.7549, p < 0.0001; 19 locations). Within the Mediterranean, no significant correlation was found between genetic and geographic distances (r = -0.3210, p = 0.0954; S1 Fig).
Fig 3. Neighbor-Net dendrogram built from Reynolds distances between 26 samples.
Sample abbreviations are as defined in Table 1.
With respect to the analysis of individual clustering using the STRUCTURE software, when 2 group clusters were considered (K = 2) a clear distinction could be observed between the samples from the Mediterranean Sea and the others (Fig 4). In any event, the best K value obtained was 3 (S2A Fig), which clearly distinguished the Pacific Ocean (red) samples from those of the Atlantic Ocean (mostly green; Fig 4). The case of samples from the Indian Ocean is special in that we observed intermediate percentages of the components of the Atlantic and the Pacific. In the same way, the GENELAND software also detected K = 3 as the most probable number of groups (S2B Fig). In this analysis, the 3 clusters were made up of the Mediterranean samples (cluster 1), the Atlantic samples (cluster 2) and the Indo-Pacific samples (cluster 3). When STRUCTURE analysis was repeated for each of the major clusters, no structure was detected within them, since the best K value obtained was 1 for all the analysis.
Fig 4. Individual clustering analysis implemented with STRUCTURE software for K = 2 and K = 3.
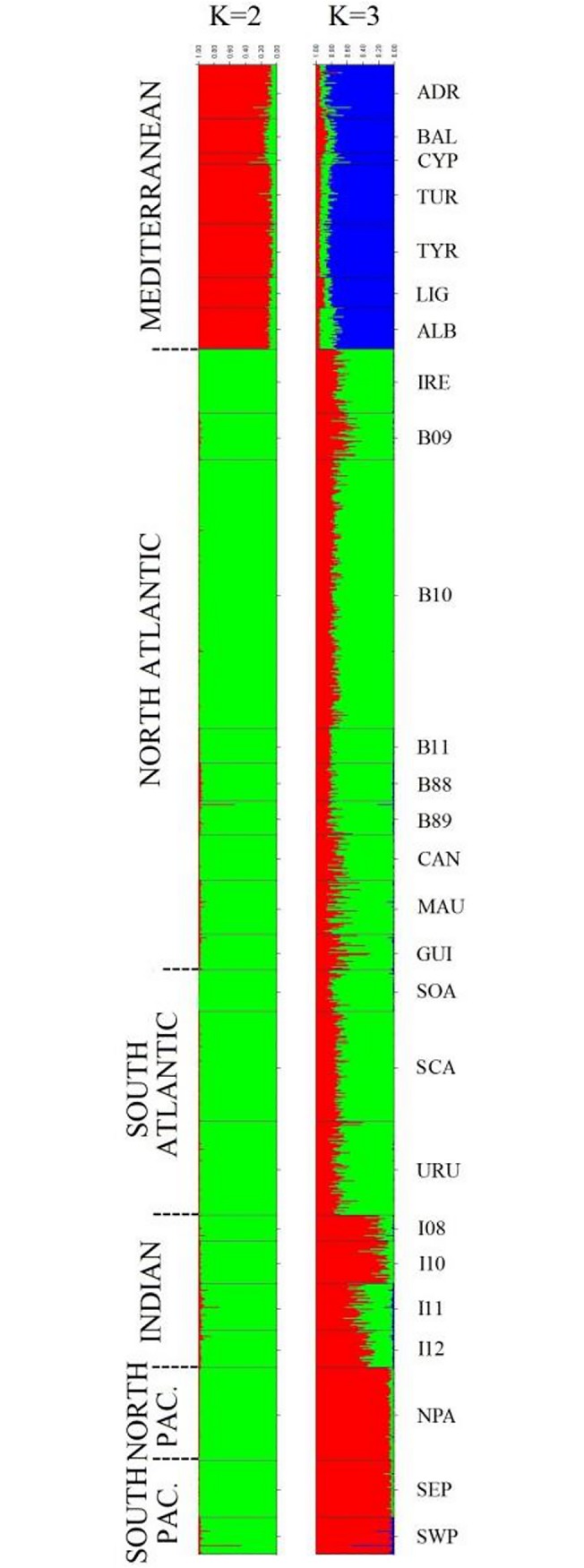
Each vertical bar represents an individual. The 26 locations are separated by horizontal continuous black lines, and the currently accepted 6 stocks are separated by discontinuous horizontal black lines. The color proportions of each bar correspond to individuals’ estimated membership fractions of each of the clusters. Sample abbreviations are as defined in Table 1.
A total of 17 out of the 75 independent markers were identified as outliers, therefore, 58 SNPs were defined as neutral SNPs. Heterogeneity analyses performed within stocks based on the 58 neutral SNPs revealed that the 6 stocks defined by the Regional Fisheries Management Organizations (RFMOs) were genetically homogeneous (p > 0.05; Table 2). POWSIM simulations showed that the 50 SNPs with the highest FST values together were able to detect significant differences among samples with FST = 0.0015 in about 95% of the tests, and with FST = 0.002 in 100% of the tests (Table 3). The FST values between stocks varied from a minimum FST = 0.001 between the North and South Atlantic and between the North and South Pacific, and a maximum FST = 0.051 between the South Pacific and the Mediterranean stock. All comparisons were found to be statistically significant, except those obtained between the North and South Atlantic, the North and South Pacific and between the North Pacific and the Indian Ocean (Table 2).
Table 2. Pairwise FST values (below the diagonal) and p-values (above the diagonal) between the 6 stocks currently recognized by the RFMOs.
| MED | NATL | SATL | IN | NPAC | SPAC | |
|---|---|---|---|---|---|---|
| MED | 0.003 | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* |
| NATL | 0.035* | 0.004 | 0.783 | <0.001* | <0.001* | <0.001* |
| SATL | 0.033* | 0.001 | 0.000 | <0.001* | <0.001* | <0.001* |
| IN | 0.038* | 0.010* | 0.008* | 0.002 | 0.125 | 0.038* |
| NPAC | 0.049* | 0.025* | 0.022* | 0.002 | - | 0.405 |
| SPAC | 0.051* | 0.026* | 0.023* | 0.003* | 0.001 | 0.004 |
Stock abbreviations: MED (Mediterranean), NATL (North Atlantic), SATL (South Atlantic), IN (Indian), NPAC (North Pacific) and SPAC (South Pacific). FST values among locations within stocks are shown on the diagonal, and none of them were significant (p-value > 0.05).
* significant p-value (<0.001)
Table 3. Probability of detecting a particular level of differentiation (FST) among populations.
| FST | Pχ 2 |
|---|---|
| 0.0005 | 0.351 |
| 0.0015 | 0.952 |
| 0.0020 | 0.999 |
| 0.0025 | 1.000 |
| 0.0050 | 1.000 |
Regarding adaptation of the populations to the specific environmental conditions of their surroundings, the 17 markers identified as outliers using BAYESCAN were analyzed. The defined haplotype ss974292126+ss974292127 had a positive alpha value and significant high FST value, suggesting that it may be subject to divergent selection [60]. This haplotype was practically monomorphic in non-Mediterranean samples (fCC = 0.999 and fCA = 0.001), while haplotype frequencies in the Mediterranean Sea were significantly different (fTA = 0.127; fCC = 0.819; fTC = 0.016; fCA = 0.038; FST = 0.311; p < 0.0001). The remaining 16 outlier SNPs showed a negative value for alpha and significant low FST values, a result consistent with balancing selection (S3 Fig). Candidate genes involved in essential metabolic pathways were found by homology between our sequence data surrounding the 16 outlier SNPs, and previously known teleost genes (S2 Table).
Effective population size
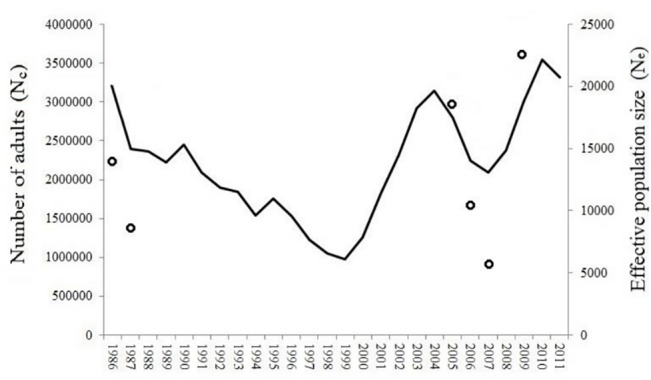
Effective population size (Ne) was estimated for the North Atlantic Ocean analyzing the 58 neutral SNP markers. While short-term Ne ranged between 5,466 and 23,330 (C07 and C08 cohorts, respectively) (mean short-term Ne = 13,267 ± 6,049; S3 Table), long‐term Ne varied between 13,897 and 20,304 (C08 and C06 cohorts, respectively) (mean long‐term Ne = 16,729 ± 2,248;S4 Table). Mean short- and long-term Ne were not significantly different (Mann-Whitney U, p-value > 0.05). The short-term Ne was compared to Nc (Fig 5), and Ne/Nc ratio (ratio of effective-to-census size) values were found to range between 2.62 × 10–3 and 9.83× 10–3 (C07 and C08 cohorts, respectively). Despite the apparent correlation between Ne and Nc, it was not found to be statistically significant (r = 0.383; p-value = 0.453).
Fig 5. Estimates of the evolution of the number of adults from 1986 to 2011 (solid line, Nc), and the short-term effective population size of each cohort in the North Atlantic (dots, Ne).
Discussion
A sustainable management of fisheries requires the exploitation of one single population per stock, and accurate population size estimates [41]. In this way, two problems that reduce intraspecific diversity are avoided: overexploitation and the risk of losing minority populations when various populations are managed as a single stock. The present study is the most comprehensive genetic study carried out to date of the albacore species worldwide. Overall, 117 novel nuclear SNPs were applied to 1,331 albacore individuals from 26 locations covering the whole distribution area of the species. We have described the genetic structure of the species, provided effective population size estimates for the North Atlantic Ocean population, and reported putative signs of natural selection in the albacore genome. Results obtained indicated that none of the currently defined 6 management units includes more than one genetic population. Regarding population size, Ne estimates ruled out the occurrence of severe historical bottlenecks in the North Atlantic Ocean population, and showed that current levels of genetic diversity are sustainable over the time, thereby corroborating the resiliency and responsiveness of the albacore. All these results on albacore population genetic characteristics should contribute to more rational and sustainable fisheries management policies and programs for this important fish species.
Cross-species amplification of SNPs
This study has shown that cross-species amplification is a valuable approach to identify SNP markers in the albacore species, with a final validation rate of 24.61%. The reciprocal cross was reported by Albaina et al. [34], who showed that albacore and the ABFT species shared 18% of SNPs. Cross-amplification success between Atlantic herring (Clupea harengus) and Pacific herring (Clupea pallasii) is even lower, 12% [74]. The higher success obtained in the present study lies in the high number of individuals and the assortment of their geographical origins. Here, 1,331 albacore individuals worldwide were studied, whereas four Pacific herrings were analyzed by Helyar et al. [74] and 107 Atlantic bluefin tunas by Albaina et al. [34]. Cross-amplification success also relies on the design of an appropriate SNP set, such as the 384 SNPs from coding regions analyzed in this study. In conserved regions of the genome, such as coding regions, the similarity between the analyzed sequences of two species is increased and therefore, the chance to share SNPs also increases.
Cross-species amplification is considered a valuable approach to identify SNP markers in non-model organisms. Additionally, when these SNPs are located in genes, as is the case in the present study, they can be used for local adaptation studies. In this regard, ss974292126+ss974292127 SNP haplotype was identified as an outlier in BAYESCAN analysis, being highly polymorphic in the Mediterranean while nearly fixed in the three oceans. The result obtained for the ss974292126+ ss974292127 SNP haplotype in the present study is indicative of a clear pattern of diversifying selection. From an adaptive point of view, this result depicts an environmental scenario in the Mediterranean different with respect to the environmental homogeneity of the three oceans. Unfortunately, no homology was found to known teleost’s genes (S2 Table). We also found 16 outlier SNPs with an anomalous homogeneity within the species. One likely scenario is balancing selection actively maintaining those SNPs in the gene pool of albacore. In fact, the inspection of the sequences surrounding the 16 outlier SNPs revealed 13 candidate genes involved in essential metabolic pathways. However, alternative explanations cannot be ruled out: such outliers could also reflect reduced variation at these loci if the minor allele was rare in all populations around the globe, or they could even be false positives. The fast growing genomic data base in the marine world will help to decipher these findings in the near future.
Population genetic structure
The genetic structure revealed in the present study is reliable since it was based on quite a large sample size, and on an extensive spatial and temporal distribution of samples. Moreover, POWSIM simulations showed that the 50 SNPs with the highest FST values together yielded a type II error rate (failure to detect a real difference) of 0% for divergences of FST = 0.002 or greater. In all, 4 spatial-temporally homogeneous populations were identified for the albacore species: Mediterranean Sea, Atlantic Ocean, Indian Ocean, and Pacific Ocean populations (Table 2). When STRUCTURE analysis was repeated for each of the major clusters (Mediterranean, Pacific and Atlantic), no structure was detected within them. This result bears out the findings of Albaina et al. [34]. On the contrary, the genetic heterogeneity within the Mediterranean suggested by others using microsatellite markers [32,33], the observation of separate spawning grounds [39], or differences in isotopic composition [75], was not detected using SNPs. Within the Mediterranean no significant correlation was found between genetic and geographic distances. Present findings also contradicted previously suggested heterogeneity for the Pacific Ocean using microsatellite markers [29,33] or studying migrations, spawning areas and seasons as criteria [76]. And lastly, the Atlantic Ocean was found to be homogeneous in terms of genetic structure, thus challenging earlier results and interpretations on the basis of blood groups [7], microsatellites [29,32] and migratory features [6,77]. In fact, albacore tuna tagging experiments are very scarce, specially in the South Atlantic, Indian Ocean and throughout the Mediterranean Sea. Thus, there is little information about their migratory behavior and the data available are not very informative about population structure and mixing. Moreover, unfortunately, there is little knowledge about albacore spawning areas and times for the different populations [15], and this makes it difficult to get reference samples of known origin (e.g. larvae or young of the year) for genetic studies. Using samples that might represent transient migrants could, in principle, provide a misleading picture of population structure (e.g. suggesting homogeneity within the Mediterranean, where some structure might exist). This potential problem affects mostly at small scales and less at the scale of ocean basins and/or hemispheres. New knowledge about albacore spawning areas and seasons, as well as increased access to reference samples will allow to design more robust genetic experiments to reveal population structure and mixing at smaller scales.
With respect to the discrepancies between the present study and those using microsatellites, and in regard to the power of the markers, although an individual SNP show less power than do multi-allelic microsatellite loci [78], 4–12 nuclear SNPs are expected to have the same power as a single microsatellite locus [79]. Moreover, SNP markers have advantages over other markers: the use of single-tube multiplex assays with small PCR products (60–80 bp) could potentially produce better quality data more efficiently than would genotyping multiple microsatellites, and using SNP loci lies in a more representative sample of the entire genome and a reduced interlocus sampling variance [80].
In this study, neutral SNP variation (Table 2, Figs 3 and 4) and the SNP haplotype putatively under selection showed the Mediterranean group as the most differentiated from the rest of populations. Similarly, extremely different frequencies for G6PD locus and mtDNA D-Loop sequences between Mediterranean and Atlantic samples were described by Nakadate et al. [31], and interpreted as indicative of a restricted gene flow. Our results agreed with this, since FST values between Mediterranean and the rest of populations are the highest, ranging between 0.033 and 0.051 (Table 2). The isolation of the Mediterranean population contrasted with the higher gene flow that occurs between the three Oceans. In fact, when the three Oceans were analyzed together, correlation between genetic and geographic distances of the different sampling points were found (r = 0.7549; p-value < 0.0001; S1B Fig), as previously described for the Atlantic herring, another migratory pelagic marine fish [81]. Although there is great evidence that other species (such as Atlantic bluefin tuna and swordfish (Xiphias gladius) [82,83,84]) migrate substantially across Strait of Gibraltar, migration is negligible for albacore [6,31]. Results obtained in the present study, together with those using different methodologies, such as genetic markers [30–34], growth parameters [85] and tagging experiments [6] confirm the singularity of the Mediterranean albacore. It is difficult to evaluate whether this singularity is due only to current restricted gene flow, or it may reflect also the demographic history of Mediterranean albacore. According to Kettle et al., [86] the Mediterranean would have served as a refugia for a range of marine species during the last glacial maximum (LGM). Under this latter hypothesis, Mediterranean population would be the result of one major founding event, and would have been isolated from all other populations for a long time. A similar scenario has been proposed for Atlantic herring in the Baltic Sea [87]. In order to shed light on the controversial genetic relationship of the Indian albacore population with that of the Atlantic or Pacific, we analyzed an ample sample including 774 individuals from 12 Atlantic locations, 167 from three Pacific locations, and 136 individuals from four localities in the Western Indian Ocean. Results indicated that Indian samples appeared genetically closer to North Pacific ones, since the FST value between these populations was the only no significant comparison (Table 2). Our work thus confirms with a large sample of the Indian albacore population the results of Albaina et al. [34], who analyzed, also with SNPs, 24 individuals. This sample was the same as that used in the study with microsatellites by Montes et al. [33], although different results were obtained in both studies, since the analysis with 8 microsatellite markers indicated that the Indian albacore population was closer to the Atlantic than to the Pacific one. We think that in this case results may be biased due to the analysis of highly polymorphic markers in a small sample. In any case, the present study also detected that Indian albacore showed both Atlantic and Pacific components in STRUCTURE (Fig 4) and GENELAND analyses. That is, Cape of Good Hope did not represent a definitive barrier to gene flow, as it has been described by other authors [6,28,33].
Effective population size
Albacore is an overexploited species, whose biomass started decreasing due to overfishing 3 decades ago. Tuna stock assessments based on fishery data are highly uncertain (see [88]) and albacore is not an exception [40,89]. Albacore is a species with seemingly large populations, however they could be more sensitive to genetic drift and inbreeding from intensive harvests than census sizes would suggest [90,91]. In these cases, management requires the maintenance of a much larger census size than would typically be recommended on the basis of information about population dynamics [91]. This is an assumed problem associated to overfished populations: that the high fishing pressure leads to genetic bottlenecks [92,93]. If true, this could have serious implications for management procedures [90,91,94]. Therefore, estimating of Ne for sustainable management purposes is a good choice, because it integrates genetic effects with the life history of the species, allowing for predictions of a population's current and future viability [91]. Our analyses on population genetic structure showed no statistically significant spatial or temporal fluctuations within each of the four defined populations. This result indicated that (1) migration had failed to alter allele frequencies at each region, and that (2) the effective population size in each region was large enough to prevent microdifferentiation processes driven by genetic drift. This latter hypothesis was supported for the North Atlantic Ocean population; similar short- and long-term Ne estimates for this population suggested that in spite of the fishing impact on biomass (Nc), genetic diversity remains high and, therefore, viability of the population has not been affected, this is, it has not suffered severe historical bottlenecks.
From a fishery management perspective, short-term Ne estimates could provide an approach for generating a fishery-independent indicator of population status. Temporal variations in such an indicator could serve as a prognostic marker of the genetic diversity of exploited albacore tunas and trigger specific well planned management responses to signs of reduced diversity (e.g. drastic reduction of fishing effort until genetic diversity is recovered). Management must often default to apparently simple rules-of-thumb, such as the 50/500 criteria for maintenance of genetic diversity; this means that a short-term Ne ≥ 50 is required to avoid the damaging effects of inbreeding, and a short-term Ne ≥ 500 is necessary to avoid extinctions due to the inability to evolve to cope with environmental change. Taking this rule into account, we have demonstrated that albacore population size in the North Atlantic Ocean is high enough for dealing with both, inbreeding effects and adaptation capabilities. But for management purposes, Ne estimates might be more adequate to better understand how ecological factors reduce or increase the Ne/Nc ratio. With this regard, theory suggests that Ne/Nc ratios in the wild should be above 0.1 [95–97], and empirical evidence for several wild populations of different non marine species is consistent with this prediction, showing Ne/Nc ratios ranging from 0.10 to 0.14 [96,98]. In the North Atlantic Ocean, the effective population size was three orders of magnitude lower than the adult census size (S3 Table). These figures are within the range documented for other fish species, such as Sciaenops ocellatus [99], Pagrus auratus [92] and Sebastes crameri [100]. A low Ne/Nc ratio could be explained by variance in albacore survival due to high larval and pre-recruit mortality [101,102], indicating that few mature adults contribute to each generation. It has been questioned the appropriateness of estimating Ne from temporal data in species with high effective population sizes [90], and whether Ne/Nc ratios reflect the true dynamics of biological systems [94,103]. Nevertheless, it is important to obtain a better understanding of how vulnerable fish populations are to loss of genetic variation and in that respect, the data presented here on temporal stability at neutral markers will serve as an important baseline for future evaluations of Ne/Nc and for monitoring Ne in albacore. In conclusion, Ne estimate, as a fishery-independent index of abundance, provides a valuable complementary tool for monitoring the status of fish populations in order to implement more sustainable management actions.
Supporting Information
a) Regression of pairwise geographic distance and genetic similarity for the 26 locations. b) Similar analysis using 19 locations from the Atlantic, Indian and Pacific Oceans and c) considering only the 7 Mediterranean samples.
(TIFF)
(a) Mean probabilities of the data [LnPr(X)|K] over 10 STRUCTURE replicated runs plotted as a function of putative number of clusters (K). (b) Posterior density distribution of the number of estimated clusters.
(TIFF)
Graphical representation of the markers based on FST values (y axis) against log(q-value) (x axis). Candidate markers under selection are those with a q-value less than 0.1, represented at the right of the vertical black line. Grey circles represent candidate markers for balancing selection and black circles represent candidate marker for divergent selection; empty circles represent putatively neutral loci.
(TIFF)
(*) SNPs obtained from Albaina et al. [34].
(TIFF)
(TIFF)
Generation time (Ĝ), Fs values, harmonic means of effective (Ňe), spawning census population size (Ňc), and Ňe/Ňc ratio.
(TIFF)
Number of samples representing each cohort (N), spawning census population size (Nc) and Ne/Nc ratio values are listed.
(TIFF)
Acknowledgments
The authors are indebted to technical and human support provided by General Research Services SGIker in the University of the Basque Country (UPV/EHU). We are grateful to the following institutes who provided samples: South African Institute for Aquatic Biodiversity (SAIAB, South Africa), Galway-Mayo Institute of Technology (GMIT, Ireland), Instituto Español de Oceanografía (IEO, Spain). We also are grateful to E. Jimenez, M.A. Pardo, I. Fraile, I. Arregi, J. Lopez, J. Filmarter, J. Areso, A. Delgado, D. Brophy, K.Schaefer, D. Fuller and V. Allain for sampling assistance. This research was supported by the projects ATM2010Hegaluze (351BI20090047) and TUNASNIP (SPE10UN92) funded by the Basque Government. Urtzi Laconcha’s work was supported by a PhD grant by the Fundación Centros Tecnológicos Iñaki Goenaga. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.). This manuscript was improved thanks to the comments of two anonymous reviewers. We express our gratitude to ICCAT for funding the identification of ABFT SNPs (GBYP PHASE2 project), and to GBYP consortium members for providing them for cross-amplification.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the projects ATM2010 Hegaluze (351BI20090047) and TUNASNIP (SPE10UN92) funded by the Basque Government. Urtzi Laconcha’s work was supported by a PhD grant by the Fundación Centros Tecnológicos Iñaki Goenaga. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Arrizabalaga H, Dufour F, Kell L, Merino G, Ibaibarriaga L, Chust G, et al. (2014) Global habitat preferences of commercially valuable tuna. Deep-Sea Res Pt II: Topical Studies in Oceanography.
- 2. FAO 2014. Fisheries and aquaculture software. FishStatJ—software for fishery statistical time series In: FAO Fisheries and Aquaculture Department; [online]. Rome: Updated 22 July 2014. Available: http://www.fao.org/fishery/statistics/software/fishstatj/en. [Google Scholar]
- 3. Arrizabalaga H, Lopez-Rodas V, Oritz de Zarate V, Costas E, González-Garcés A (2002) Study on the Migrations and Stock Structure of Albacore (Thunnus alalunga) from the Atlantic Ocean and the Mediterranean Sea Based on Conventional Tag Release- Recapture Experiences. ICCAT Col Vol Sci Pap 54: 1479–1494. [Google Scholar]
- 4. Childers J, Snyder S, Kohin S (2011) Migration and behavior of juvenile North Pacific albacore (Thunnus alalunga). Fish Oceanogr 20: 157–173. [Google Scholar]
- 5. Farley JH, Williams AJ, Hoyle SD, Davies CR, Nicol SJ (2013) Reproductive Dynamics and Potential Annual Fecundity of South Pacific Albacore Tuna (Thunnus alalunga). PLoS ONE 8(4): e60577 10.1371/journal.pone.0060577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arrizabalaga H, Costas E, Juste J, Gonzalez-Garcés A, Nieto B, Lopez-Rodas V (2004) Population structure of albacore Thunnus alalunga inferred from blood groups and tag-recapture analyses. Mar Ecol-Prog Ser 282: 245–252. [Google Scholar]
- 7. Duclerc J, Sacchi J, Piccinetti C, Piccinetti Manfrin G, Dicenta A, Barrois JM (1973) Nouvelles donne sur la reproduction du thon rouge (Thunnus thynnus L.) et d’autres espèces de Thonidés en Méditerranée. Rev Trav Inst Peches marit 37: 2. [Google Scholar]
- 8. Lalami Y, Tellai S, Barrois JM, Piccinetti C, Piccinetti Manfrin G (1973) Observations sur les oeufs et larves des thonides des cotes algeriennes. Pelagos IV (2): 54–65. [Google Scholar]
- 9. Dicenta A, Piccinetti C, Piccinetti Manfrin G (1975) Observaciones sobre la reproducción de los túnidos en las islas Baleares. Bol Inst Espa Oceanogr 204: 25–37. [Google Scholar]
- 10. Beardsley GL (1969) Proposed Migrations of Albacore, Thunnus alalunga, in the Atlantic Ocean. T Am Fish Soc 98(4): 589–598. [Google Scholar]
- 11. Carlsson J, McDowell JR, Carlsson JEL, Graves JE (2007) Genetic Identity of YOY Bluefin Tuna from the Eastern and Western Atlantic Spawning Areas. J Hered 98(1): 23–28. [DOI] [PubMed] [Google Scholar]
- 12. Koto T (1969) Studies on the albacore-XIV. Distribution and movement of the albacore in the Indian and the Atlantic Oceans based on the catch statistics of Japanese tuna long-line fishery. Bull Far Seas Fish Res Lab, Japan 1:115–129 (in Japanese with English abstract). [Google Scholar]
- 13.Shiohama T (1985) Overall fishing intensity and length composition of albacore caught by long line fishery in the Indian Ocean, 1952–1982. IPTP TWS/85/22 91–109.
- 14. Nakamura H (1969) Tuna distribution and migration. Fishing News Ltd, London: [Google Scholar]
- 15. Ueyanagi S (1969) Observations on the distribution of tuna larvae in the indo-pacific Ocean with emphasis on the delineation of the spawning areas of albacore, Thunnus alalunga . Bull Far Seas Fish Res Lab 2: 177–256. [Google Scholar]
- 16. Sund PN, Blackburn M, Williams F (1981) Tunas and their environment in the Pacific Ocean: A review. Oceanogr. Mar. Biol. 19: 443–512 [Google Scholar]
- 17. Ward RD (2000) Genetics in fisheries management. Hydrobiologia, 420: 191–201. [Google Scholar]
- 18. Waples RS, Gaggiotti O (2006) Invited review: What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Mol Ecol 15: 1419–1439. [DOI] [PubMed] [Google Scholar]
- 19. Ricker WE (1981) Changes in the average size and average age of Pacific salmon. Can J Fish Aquat Sci 38: 1636–1656. [Google Scholar]
- 20. Pawson MG, Jennings S (1996) A critique of methods for stock identification in marine capture fisheries. Fish Res 25: 203–217. [Google Scholar]
- 21. Waldman JR (1999) The importance of comparative studies in stock analysis. Fish Res 43: 237–246. [Google Scholar]
- 22. Miller JA, Gray A, Merz J (2010) Quantifying the contribution of juvenile migratory phenotypes in a population of Chinook salmon Oncorhynchus tshawytscha . Mar Ecol-Prog Ser 408: 227–240. [Google Scholar]
- 23. García A, Cortés D, Ramírez T, Fehri-Bedoui R, Alemany F, Rodríguez JM, et al. (2006) First data on growth and nucleic acid and protein content of field-captured Mediterranean bluefin (Thunnus thynnus) and albacore (Thunnus alalunga) tuna larvae: a comparative study. Sci Mar 70: 67–78. [Google Scholar]
- 24. Macdonald JI, Farley JH, Clear NP, Williams AJ, Carter TI, Davies CR, et al. (2013) Insights into mixing and movement of South Pacific albacore Thunnus alalunga derived from trace elements in otoliths. Fish Res 148: 56–63. [Google Scholar]
- 25.Bard FX (1981) La thon germon (Thunnus alalunga) de l’Ocean Atlantique. De la dynamique de population à la stratégie démographique. Thèse Doctorat ès Sciences Naturelles, Universitè de Paris VI, 330P.
- 26. Suzuki A (1962) Serologocal studies of the races of tuna. VI. Bigeye-3 antigen occurred in the albacore. Rep Nankai Reg Fish Res Lab 16:67–70 (in Japanese with English abstract) [Google Scholar]
- 27. Graves JE, Dizon AE (1989) Mitochondrial DNA sequence similarity of Atlantic and Pacific albacore tuna (Thunnus alalunga). Can J Fish Aquat Sci 46: 870–873. [Google Scholar]
- 28. Chow S, Ushiama H (1995) Global population structure of albacore (Thunnus alalunga) inferred by RFLP analysis of the mitochondrial ATPase gene. Mar Biol 123: 39–45. [Google Scholar]
- 29. Takagi M, Okamura T, Chow S, Taniguchi N (2001) Preliminary study of albacore (Thunnus alalunga) stock differentiation inferred from microsatellite DNA analysis. Fish Bull 99: 697–701. [Google Scholar]
- 30. Viñas J, Alvarado Bremer JR, Pla C (2004) Inter-oceanic genetic differentiation among albacore (Thunnus alalunga) populations Mar Biol 145: 225–232. [Google Scholar]
- 31. Nakadate M, Viñas J, Corriero A, Clarke S, Suzuki N, Chow S (2005) Genetic isolation between Atlantic and Mediterranean albacore populations inferred from mitochondrial and nuclear DNA markers. J Fish Biol 66: 1545–1557. [Google Scholar]
- 32. Davies CA, Gosling EM, Was A, Brophy D, Tysklind N (2011) Microsatellite analysis of albacore tuna (Thunnus alalunga):population genetic structure in the North-East Atlantic Ocean and Mediterranean Sea. Mar Biol 158: 2727–2740. [Google Scholar]
- 33. Montes I, Iriondo M, Manzano C, Arrizabalaga H, Jiménez E, Pardo MA, et al. (2012) Worldwide genetic structure of albacore (Thunnus alalunga) revealed by microsatellite DNA markers. Mar Ecol-Prog Ser 471: 183–191. [Google Scholar]
- 34. Albaina A, Iriondo M, Velado I, Laconcha U, Zarraonaindia I, Arrizabalaga H, et al. (2013) Single nucleotide polymorphism discovery in albacore and Atlantic bluefin tuna provides insights into worldwide population structure. Anim Genet 44: 678–692. 10.1111/age.12051 [DOI] [PubMed] [Google Scholar]
- 35. Fontaine MC, Baird SJE, Piry S, Ray N, Tolley KA, Duke S, et al. (2007) Rise of oceanographic barriers in continuous populations of a cetacean: the genetic structure of harbour porpoises in Old World waters. BMC Biol 5: 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riccioni G, Landi M, Ferrara G, Milano I, Cariani A, Zane L, et al. (2010) Spatio-temporal population structuring and genetic diversity retention in depleted Atlantic bluefin tuna of the Mediterranean Sea. P Natl A Sci 107: 2102–2017. 10.1073/pnas.0908281107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jansen T, Gislason H (2013) Population Structure of Atlantic Mackerel (Scomber scombrus). PLoS ONE 8(5): e64744 10.1371/journal.pone.0064744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pujolar JM, Roldan MI, Pla C (2003) Genetic analysis of tuna populations, Thunnus thynnus and T. alalunga . Mar Biol 143: 613–21. [Google Scholar]
- 39. Marano G, De Zio V, Pastorelli AM, Rositani L, Ungaro N, Vlora A (1999) Studio sinottico sulla biologia e pesca di Thunnus alalunga (Bonnaterre, 1788). Biol Mar Medit 6: 192–214. [Google Scholar]
- 40.ICCAT 2014. Report of the 2013 ICCAT North and South Atlantic albacore stock assessment meeting (Sukarrieta, Spain—June 17 to 24, 2013). ICCAT Collective Volume of Scientific Papers. In press.
- 41. Hauser L, Carvalho GR (2008) Paradigm shifts in marine fisheries genetics: ugly hypotheses slain by beautiful facts. Fish Fish 9: 333–362. [Google Scholar]
- 42. O'Leary SJ, Hice LA, Feldheim KA, Frisk MG, McElroy AE, Fast MD, et al. (2013) Severe Inbreeding and Small Effective Number of Breeders in a Formerly Abundant Marine Fish. PLOS One 8: e66126 10.1371/journal.pone.0066126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bravington MV, Grewe PM, Davies CR (2014) Fishery-independent estimate of spawning biomass of Southern Bluefin Tuna through identification of close-kin using genetic markers. FRDC Report 2007/034. CSIRO, Australia.
- 44. Nomura S, Kobayashi T, Agawa Y, Margulies D, Scholey V, Sawada Y, et al. (2014) Genetic population structure of the Pacific bluefin tuna Thunnus orientalis and the yellowfin tuna Thunnus albacares in the North Pacific Ocean. Fisheries Sci 80: 1193–1204. [Google Scholar]
- 45. Qiu F, Miyamoto MM (2011) Use of nuclear DNA data to estimate genetic diversity and population size in Pacific Bluefin and Yellowfin Tuna (Thunnus orientalis and T. albacares). Copeia 2011: 264–269. [Google Scholar]
- 46. Qiu F, Kitchen A, Beerli P, Miyamoto MM (2013) A possible explanation for the population size discrepancy in tuna (genus Thunnus) estimated from mitochondrial DNA and microsatellite data. Mol Phylogenet Evol 66: 463–468. 10.1016/j.ympev.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 47. Santiago J, Arrizabalaga H (2005) An integrated growth study for North Atlantic albacore (Thunnus alalunga Bonn. 1788) ICES Journal of Marine Science: Journal du Conseil 62: 740–749. [Google Scholar]
- 48. Rousset F (2008) Genepop’007: a complete reimplementation of the Genepop software for Windows and Linux. Mol Ecol Resour 8: 103–106. 10.1111/j.1471-8286.2007.01931.x [DOI] [PubMed] [Google Scholar]
- 49. Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68: 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reynolds J, Weir BS, Cockerham CC (1983) Estimation of the coancestry coefficient: basis for a short-term genetic distance. Genetics 105: 767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langella O (2002) Populations 1.2.32. CNRS UPR 9034.
- 52. Hudson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23: 254–267. [DOI] [PubMed] [Google Scholar]
- 53. Rousset F. (1996) Equilibrium values of measures of population subdivision for stepwise mutation processes. Genetics 142: 1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jensen JL, Bohonak AJ, Kelley ST (2005) Isolation by distance, web service. BMC Genet 6:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guillot G, Santos F, Estoup A (2008) Analysing georeferenced population genetics data with Geneland: a new algorithm to deal with null alleles and a friendly graphical user interface. Bioinformatics 24: 1406–1407. 10.1093/bioinformatics/btn136 [DOI] [PubMed] [Google Scholar]
- 57. Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164: 1567–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jakobsson M, Rosenberg NA (2007) CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23: 1801–1806. [DOI] [PubMed] [Google Scholar]
- 59. Rosenberg NA (2004) Distruct: a program for the graphical display of population structure. Mol Ecol Notes 4: 137–138. [Google Scholar]
- 60. Foll M, Gaggiotti OE (2008) A genome scan method to identify selected loci appropriate for both dominant and codominant markers: a Bayesian perspective. Genetics 180: 977–993. 10.1534/genetics.108.092221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 57: 289–300. [Google Scholar]
- 62. Weir BS, Cockerham CC (1984) Estimating F statistics for the analysis of population structure. Evolution 38: 1358–1370. [DOI] [PubMed] [Google Scholar]
- 63.Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3). Available: http://www2.unil.ch/popgen/softwares/fstat.htm.
- 64. Ryman N, Palm S, André C, Carvalho GR, Dahlgren TG, Jorde PE, et al. (2006) Power for detecting genetic divergence: differences between statistical methods and marker loci. Mol Ecol 15: 2031–2045. [DOI] [PubMed] [Google Scholar]
- 65. Waples RS (1989) A generalized approach for estimating effective population size from temporal changes in allele frequency. Genetics 121: 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jorde PE, Ryman N (1995) Temporal allele frequency change and estimation of effective size in populations with overlapping generations. Genetics 139: 1077–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jorde PE, Ryman N (2007) Unbiased estimator for genetic drift and effective population size. Genetics 177: 927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Waples RS, Yokota M (2007) Temporal estimates of effective population size in species with overlapping generations. Genetics 175: 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Felsenstein J (1971) Inbreeding and variance effective numbers in populations with overlapping generations. Genetics 68: 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Beerli P, Felsenstein J (2001) Maximum likelihood estimation of a migration matrix and effective population sizes in n subpopulations by using a coalescent approach. P Natl A Sci 98: 4563–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gemmell NJ, Schwartz MK, Robertson BC (2004) Moa were many. P Roy Soc Lond B Bio 271: 430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Santiago J (1993) A new length-weight relationship for the North Atlantic albacore. ICCAT Collective Volume of Scientific Papers 40: 316–319. [Google Scholar]
- 73.Beerli P. (2002) MIGRATE: documentation and program, part of LAMARC, Version 1.5. Available: http://evolution.genetics/washington.edu/lamarc.html.
- 74. Helyar SJ, Limborg MT, Bekkevold D, Babbucci M, Van Houdt J, Maes GE, et al. (2012) SNP Discovery Using Next Generation Transcriptomic Sequencing in Atlantic Herring (Clupea harengus). PLoS ONE 7: e42089 10.1371/journal.pone.0042089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Goñi N, Logan J, Arrizabalaga H, Jarry M, Lutcavage M (2011) Variability of albacore (Thunnus alalunga) diet in the Northeast Atlantic and Mediterranean Sea. Mar Biol 158: 1057–1073. [Google Scholar]
- 76.Lewis AD (1990) South Pacific albacore stock structure: a review of available information. South Pacific Commission, Noumea, New Caledonia, p 13
- 77. Ortiz de Zárate V, Cort JL (1998) Albacore (Thunnus alalunga, Bonnaterre) stock structure in the Atlantic Ocean, as inferred from distribution and migration patterns. Proceedings of the ICCAT Tuna Symposium ICCAT Tuna Symposium 1: 251–260. [Google Scholar]
- 78. Haasl R, Payseur B (2011) Multi-locus inference of population structure: a comparison between single nucleotide polymorphisms and microsatellites. Heredity 106: 158–171. 10.1038/hdy.2010.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Guichoux E, Lagache L, Wagner S, Chaumeil P, Léger P, Lepais O, et al. (2011) Current trends in microsatellite genotyping. Mol Ecol Resour 11: 591–611. 10.1111/j.1755-0998.2011.03014.x [DOI] [PubMed] [Google Scholar]
- 80. Morin PA, Luikart G, Wayne RK (2004) SNPs in ecology, evolution and conservation. Trends Ecol Evol 19: 208–216. [Google Scholar]
- 81. Ruzzante DE, Mariani S, Bekkevold D, André C, Mosegaard H, Clausen LA, et al. (2006) Biocomplexity in a highly migratory pelagic marine fish, Atlantic herring. P Roy Soc B-Biol Sci 273: 1459–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Block BA, Teo SL, Walli A, Boustany A, Stokesbury MJ, Farwell CJ, Weng KC, Dewar H, Williams TD (2005) Electronic tagging and population structure of Atlantic bluefin tuna. Nature 434: 1121–1127. [DOI] [PubMed] [Google Scholar]
- 83. Aranda G, Abascal FJ, Varela JL, Medina A. (2013) Spawning behaviour and post-spawning migration patterns of Atlantic bluefin tuna (Thunnus thynnus) ascertained from satellite archival tags. PLoS ONE 8(5): e76445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Alvarado Bremer JR, Viñas J, Mejuto J, Ely B, Pla C (2005) Comparative phylogeography of Atlantic bluefin tuna and swordfish: the combined effects of vicariance, secondary contact, introgression, and population expansion on the regional phylogenies of two highly migratory pelagic fishes. Mol Phylogenet Evol 36: 169–187. [DOI] [PubMed] [Google Scholar]
- 85. Megalofonou P (2000) Age and growth of Mediterranean albacore. J Fish Biol 57: 700–715. [Google Scholar]
- 86. Kettle AJ, Morales-Muniz A, Rosello-Izquierdo E, Heinrich D, Vøllestad LA (2011) Refugia of marine fish in the northeast Atlantic during the last glacial maximum: concordant assessment from archaeozoology and palaeotemperature reconstructions. Clim Past 7: 181–201. [Google Scholar]
- 87. Gaggiotti OE, Bekkevold D, Jørgensen HBH, Foll M, Carvalho GR, Andre C, et al. (2009) Disentangling the effect of evolutionary, demographic and environmental factors influencing genetic structure of natural populations: Atlantic herring as a case study. Evolution, 63: 2939–2951. 10.1111/j.1558-5646.2009.00779.x [DOI] [PubMed] [Google Scholar]
- 88. Fromentin JM, Bonhommeau S, Arrizabalaga H, Kell LT (2014). The spectre of uncertainty in management of exploited fish stocks: The illustrative case of Atlantic bluefin tuna. Mar Policy 47: 8–14. [Google Scholar]
- 89. Arrizabalaga H, Lopez-Rodas V, Costas E, Gonzalez-Garcés A (2007) Use of genetic data to assess the uncertainty in stock assessments due to the assumed stock structure: the case of albacore (Thunnus alalunga) from the Atlantic Ocean. Fish Bull 105: 140–146. [Google Scholar]
- 90. Poulsen NA, Nielsen EE, Schierup MH, Loeschcke V, Grønkjær P (2006) Long-term stability and effective population size in North Sea and Baltic Sea cod (Gadus morhua). Mol Ecol 15: 321–331. [DOI] [PubMed] [Google Scholar]
- 91. Hare MP, Nunney L, Schwartz MK, Ruzzante DS, Burford M, Waples RS, et al. (2011) Understanding and Estimating Effective Population Size for Practical Application in Marine Species Management. Conserv Biol 25(3): 438–449. 10.1111/j.1523-1739.2010.01637.x [DOI] [PubMed] [Google Scholar]
- 92. Hauser L, Adcock GJ, Smith PJ, Bernal Ramirez JH, Carvalho GR (2002) Loss of microsatellite diversity and low effective population size in an overexploited population of New Zealand snapper (Pagrus auratus). P Natl Acad Sci (USA) 99: 742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hutchinson WF, van Oosterhout C, Rogers SI, Carvalho GR (2003) Temporal analysis of archived samples indicates marked genetic changes in declining North Sea cod (Gadus morhua) Proc R Soc B 270: 2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Therkildsen NO, Nielsen EE, Swain DP, Pedersen JS (2010) Large effective population size and temporal genetic stability in Atlantic cod (Gadus morhua) in the southern Gulf of St. Lawrence. Can J Fish Aquat Sci 67: 1585–1595 [Google Scholar]
- 95. Nunney L, Campbell KA (1993) Assessing minimum viable population size: demography meets population genetics. Trends Ecol Evol 8: 234–239. 10.1016/0169-5347(93)90197-W [DOI] [PubMed] [Google Scholar]
- 96. Frankham R (1995) Effective population-size adult population size ratios in wildlife: A review. Genet Res 66: 95–107. [DOI] [PubMed] [Google Scholar]
- 97. Vucetich JA, Waite TA, Nunney L (1997) Fluctuating population size and the ratio of effective to census population size (Ne/N). Evolution 51(6): 2017–2021. [DOI] [PubMed] [Google Scholar]
- 98. Palstra FP, Ruzzante DE (2008) Genetic estimates of contemporary effective population size: what can they tell us about the importance of genetic stochasticity for population persistence in the wild? Mol Ecol 17: 3428–3447 [DOI] [PubMed] [Google Scholar]
- 99. Turner TF, Wares JP, Gold JR (2002) Genetic effective size is three orders of magnitude smaller than adult census size in an abundant, estuarine-dependent marine fish (Sciaenops ocellatus). Genetics 162: 1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gomez-Uchida D, Banks MA (2006) Estimation of Effective Population Size for the Long-Lived Darkblotched Rockfish Sebastes crameri . J Herd 97: 603–606. [DOI] [PubMed] [Google Scholar]
- 101. Whitlock MC, Barton NH (1997) The effective size of a subdivided population. Genetics 146: 427–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nunney L (1999) The effective size of a hierarchically structured population. Evolution 53: 1–10. [DOI] [PubMed] [Google Scholar]
- 103. Flowers JM, Schroeter SC, Burton RS (2002) The recruitment sweepstakes has many winners: Genetic evidence from the sea urchin Strongylocentrotus purpuratus . Evolution 56:1445–1453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a) Regression of pairwise geographic distance and genetic similarity for the 26 locations. b) Similar analysis using 19 locations from the Atlantic, Indian and Pacific Oceans and c) considering only the 7 Mediterranean samples.
(TIFF)
(a) Mean probabilities of the data [LnPr(X)|K] over 10 STRUCTURE replicated runs plotted as a function of putative number of clusters (K). (b) Posterior density distribution of the number of estimated clusters.
(TIFF)
Graphical representation of the markers based on FST values (y axis) against log(q-value) (x axis). Candidate markers under selection are those with a q-value less than 0.1, represented at the right of the vertical black line. Grey circles represent candidate markers for balancing selection and black circles represent candidate marker for divergent selection; empty circles represent putatively neutral loci.
(TIFF)
(*) SNPs obtained from Albaina et al. [34].
(TIFF)
(TIFF)
Generation time (Ĝ), Fs values, harmonic means of effective (Ňe), spawning census population size (Ňc), and Ňe/Ňc ratio.
(TIFF)
Number of samples representing each cohort (N), spawning census population size (Nc) and Ne/Nc ratio values are listed.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.