Table 2. Substrate scope and catalytic activity of Mb(L29A, H64V) toward carbene S–H insertion in the presence of different alkyl mercaptans and α-diazo esters.
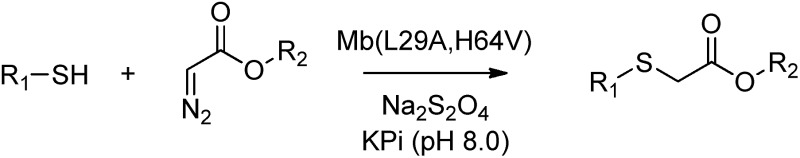
| |||||
| Entry | R1 | R2 | Product | Conv. a | TTN b |
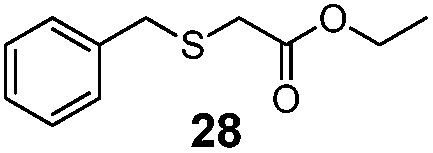
| 1 | Bn (22) | Et |
|
36% | 2550 |
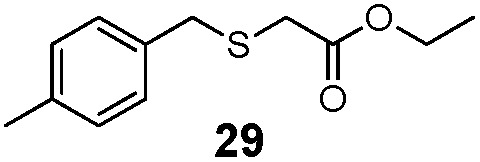
| 2 | (4-Me)PhCH2 (23) | Et |
|
51% | 2060 |
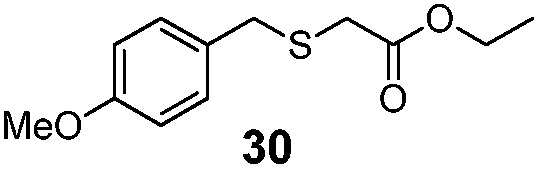
| 3 | (4-OMe)PhCH2 (24) | Et |
|
49% | 2550 |
| 4 | (4-Cl)PhCH2 (25) | Et |
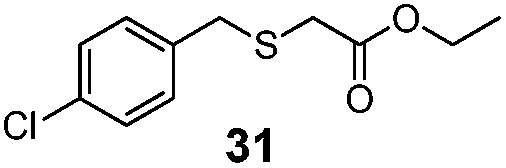
|
30% | 930 |
| 5 | C6H11 (26) | Et |
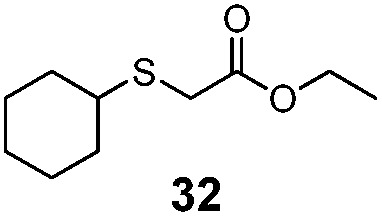
|
30% | 1100 |
| 6 | n-Octyl (27) | Et |
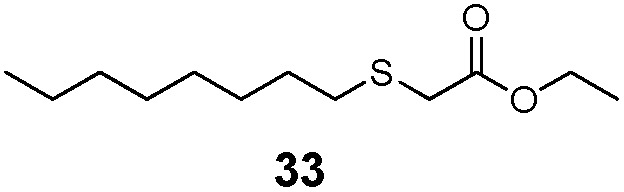
|
51% | 1730 |
| 7 | Bn (22) | Bn |
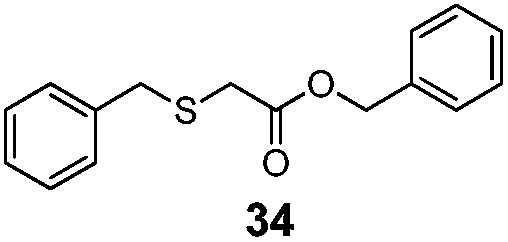
|
83% | 3050 |
| 8 | C6H11 (26) | Bn |
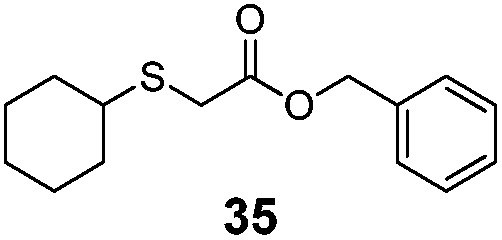
|
>99% | 4620 |
aReaction conditions: 10 mM thiol, 20 mM diazo ester, 20 μM Mb(L29A, H64V) (0.2 mol%), 10 mM Na2S2O4 in oxygen-free phosphate buffer (pH 8.0), 16 hours.
bReaction conditions: same as (a) but using 0.025 mol% protein (2.5 μM).
