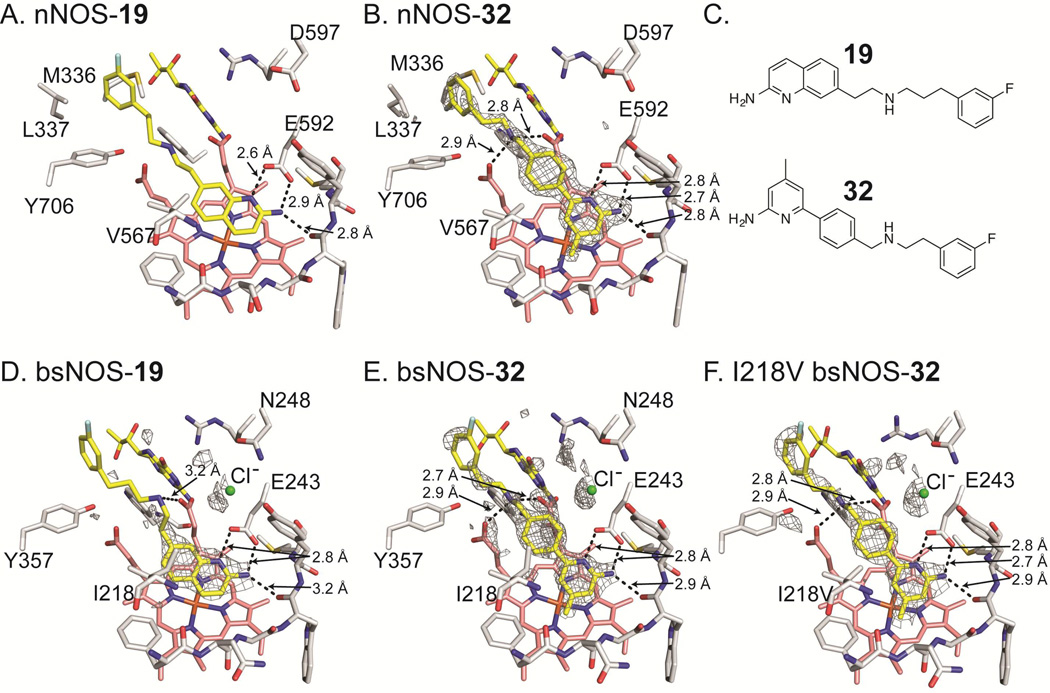
Figure 3.
Inhibitor bound NOS crystal structures with select side chains colored white, heme group colored salmon, and both the active site inhibitor and H4B molecule colored yellow. For bsNOS inhibitor bound structures there is a chlorine ion bound at the carboxylate-binding site of L-Arg, which is shown as a green sphere. Both 19 and 32 bind to nNOS and bsNOS. In the nNOS structures (A and B) the fluorinated-benzyl group binds to a hydrophobic patch that is not present in bsNOS, adjacent to the heme propionate and composed of Y706, L337 and M336. At the NOS active sites both 19 and 32 bind in similar orientations to form a network of H bonds indicated by dashed lines. For the bsNOS structures, both 19 and 32 are within a hydrophobic contact of bsNOS I218. A) 19 bound to nNOS (PDB 4CAO). B) 32 bound to nNOS with the FO-FC map contoured at 4.0σ. C) Chemical representations of 19 and 32. D) 19 bound to bsNOS with the FO-FC map contoured at 3.0σ. E) 32 bound to bsNOS with the FO-FC map contoured at 3.0σ. F) 32 bound to I218V bsNOS with the FO-FC map contoured at 3.0σ.