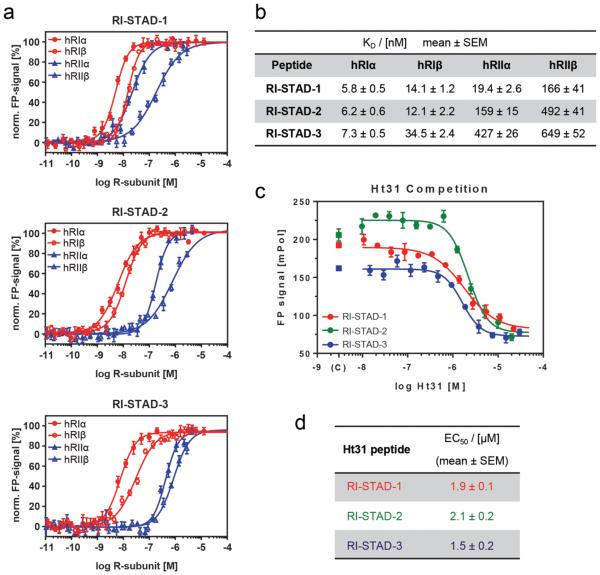
Figure 3.
In vitro characterization using full-length human R-subunits. (a) Normalized fluorescence polarization (FP) is shown for each of the full-length PKA R-subunit isoforms with the indicated fluorescently labeled peptides RI-STAD-1, -2, and -3. PKA-RI is represented in red (closed circles, α; open circles, β), and PKA-RII is shown in blue (closed triangles, α; open triangles, β). Peptides were plated at a final concentration of 4 nM, and the PKA R-subunits were tested over a concentration range of 60 pM to 10 μM. (b) Comparison of KD values of FP binding assays. RI-STAD-2 and RI-STAD-3 have higher selectivity for PKA-RI over PKA-RII. (c) FP competition spectra are shown for PKA-RIα with the three indicated RI-STAD peptides. The assay was performed using a final concentration of 4 nM RI-STADs and 5 nM RIα. The competitor peptide, Ht31, was tested over a concentration range of 15 nM to 30 μM. (d) Apparent EC50 values of FP competition assays with RIα. All FP data were collected in triplicates for each concentration measurement.