Figure 1. RIG-I-like Receptor Protein Domain Structure.
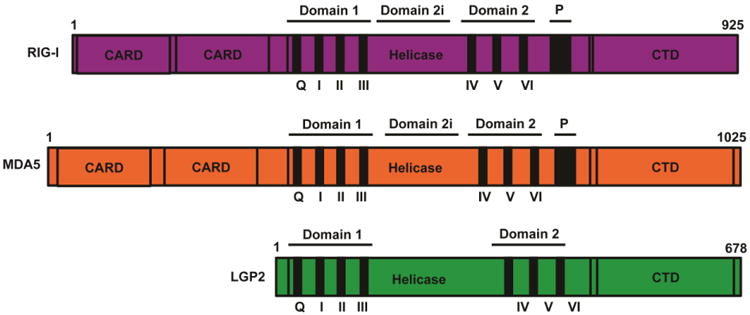
The three RLRs are SF2 type helicase proteins composed of a central DECH-box helicase domain that contains the conserved domains Hel1 (motifs Q, I, II, and III) and Hel2 (motifs IV, V, VI). Between the two helicase domains lies the insertion domain, Hel2i, required for RIG-I autoregulation. All RLRs also contain relatively conserved C-terminal domain (CTD). In RIG-I the CTD uniquely has a positively charged binding pocket for the recognition of 5′-PPP or 5′PP RNA substrates. RIG-I and MDA5 have two N-terminal caspase activation and recruitment domains (CARDs). Alignment of multiple CARDs is essential for interactions with MAVs and initiation of downstream antiviral signaling.
