Figure 2. Simplified Model: RIG-I-like Receptor Activation and Signaling.
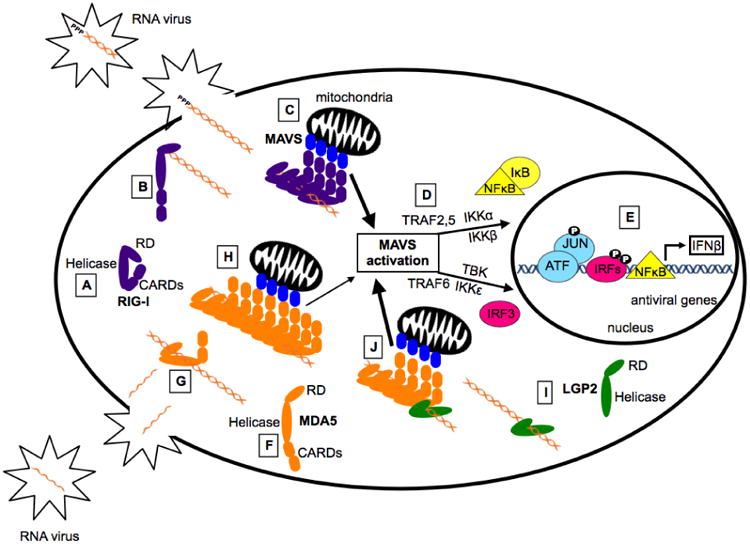
Much of our understanding of RLR signaling comes from studies characterizing RIG-I, the prototype RLR. In the cytoplasm RIG-I is present in an autoinhibited conformation (A), in which the CARDs interact with Hel2i, leaving them unavailable for downstream signaling interactions. During virus infection, non-self RNA species accumulate in the cytoplasm. RIG-I is activated when the CTD engages the appropriate non-self 5′-PPP or 5′-PP dsRNA ligand (B). This structural rearrangement transforms the helicase domain into an active ATPase and allows the formation of filamentous structures along the RNA (C). This alignment of multiple CARDs stimulates MAVS activation (D), leading to the assembly of a signaling scaffold for TRAF2, TRAF5, and TRAF6, and their associated kinases (TBK, IKKα, IKKβ, IKKε) that result in phosphorylation-mediated activation of latent IRF3 and NFκB transcription factors. These transcription factors, along with ATF/JUN, assemble on the IFNβ gene proximal enhancer and recruit RNA polymerase to induce transcription of IFNβ and other antiviral genes (E). MDA5 is present in the cytoplasm in a flexible conformation in which the CARDs are not inhibited (F). This is consistent with studies demonstrating that overexpression of MDA5 alone can stimulate the IFNβ promoter. Unlike RIG-I, MDA5 does not recognize specific RNA end modifications but binds slowly, with low affinity, internally along the dsRNA stem (G). Following nucleation, additional MDA5 monomers bind the RNA forming filaments stabilized by protein-protein interactions on adjacent MDA5 monomers (H). The alignment of MDA5 CARDs stimulates MAVS, ultimately leading to the transcription of IFNβ and other antiviral mediators. LGP2 binds with high affinity to dsRNA regardless of RNA length or end modifications, but lacks the CARDs critical for downstream signaling (I). Recent studies have demonstrated the LGP2 increases the initial rate of MDA5-RNA interaction and stabilizes the formation of shorter MDA5 filaments (J). Because LGP2 increases the rate of MDA5-RNA interaction, these shorter stable filaments are formed more rapidly and greater MAVS activation and antiviral signaling is achieved (J).
