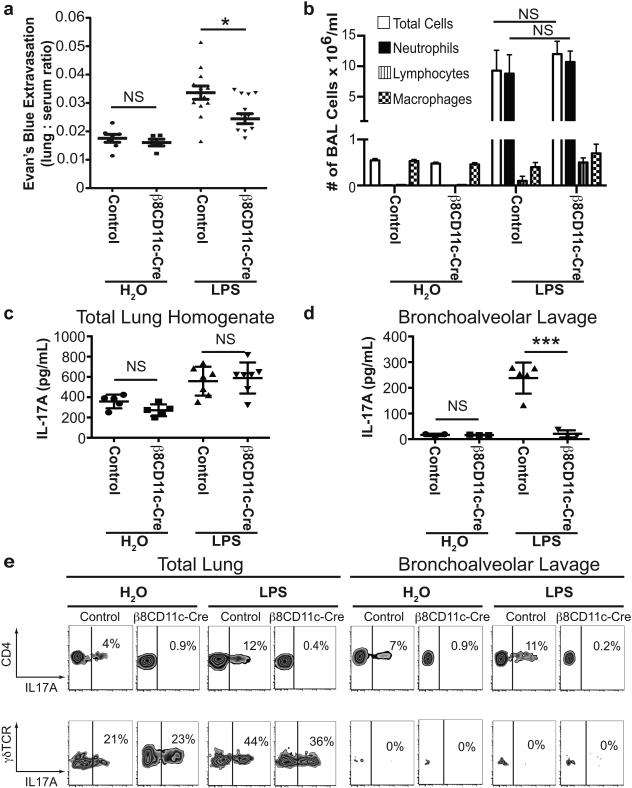
Figure 1. Itgb8fl/flxCD11c-cre mice that fail to differentiate αβTH17 cells are protected from LPS-induced increases in lung permeability.
(a) Endotracheal LPS (100 μg/mouse) or vehicle control (H2O) was administered to Itgb8fl/flxCD11c-cre and littermate control mice. Lung permeability was measured by extravasation of intravascular albumin labeled with Evan’s blue dye into the lung alveolar space and parenchyma. n=12 in Itgb8fl/flxCD11c-cre LPS group, n=13 in littermate control LPS group, n=5 in H2O groups. *p<0.05, no statistical significance (NS) = p>0.05 by one-way ANOVA and Tukey-Kramer test. (b) Total cell count and differential analysis of BAL fluid showed no difference in total cell count and neutrophil count 2 days after administration of H2O or LPS (the peak time for neutrophil influx) between Itgb8fl/flxCD11c-cre and littermate control mice. n=5 per group. NS = p>0.05, by student t-test. (c) IL-17A protein levels measured by ELISA in total lung homogenates 4 days after treatment with LPS or H2O. n=5 per group. (d) IL-17A protein levels in BAL fluid 4 days after treatment with LPS or H2O. n=3-5 per group. ***p<0.001, NS = p>0.05 by student t-test. (e) Flow cytometry analysis of total lung isolates and BAL fluid obtained 4 days after treatment with LPS or H2O. PMA and ionomycin stimulated and monensin treated cells were stained for anti-CD4, anti-γδTCR and anti-IL-17A. Top row are CD4+ gated cells and the bottom row are γδTCR+ gated cells. Data are representative flow cytometry plots with percentage of IL-17A+ cells from 4 per group. Data reported as mean ± SEM for figures a-d.