Abstract
Methamphetamine abuse impacts the global economy through costs associated with drug enforcement, emergency room visits, and treatment. Previous research has demonstrated early life stress, such as childhood abuse, increases the likelihood of developing a substance abuse disorder. However, the effects of early life stress on neuronal damage induced by binge methamphetamine administration are unknown. We aimed to elucidate the effects of early life stress on methamphetamine induced dopamine damage in the striatum. Pups were separated from dams for three hours per day during the first two weeks of development or 15 minutes for control. In adulthood, rats received either subcutaneous 0.9% saline or 5.0 mg/kg METH injections every two hours for a total of four injections. Rectal temperatures were taken before the first injection and one hour after each subsequent injection. Seven days after treatment, rats were euthanized and striatum was collected for quantification of tyrosine hydroxylase (TH) and dopamine transporters (DAT) content by western blot. Methamphetamine significantly elevated core body temperature in males and decreased striatal DAT and TH content, and this effect was potentiated by early life stress. Females did not exhibit elevated core body temperatures or changes in DAT or TH in either condition. Results indicate maternal separation increases methamphetamine induced damage, and females are less susceptible to methamphetamine induced damage.
Keywords: maternal separation, stress, methamphetamine, dopamine, striatum
I. INTRODUCTION
Methamphetamine use and abuse remains a worldwide concern. In the United States alone over 535,000 individuals meet the criteria for methamphetamine or amphetamine abuse or dependence [1]. Chronic use of methamphetamine leads to long-term decreases in dopamine transporter levels and D2 and D3 receptors in the striatum [2] and [3]. These changes correlate with cognitive deficits in the Wisconsin card sorting task and other working memory tasks [4]. Although these deficits may recover with long-term abstinence, some users still exhibit lowered transporters, receptors, and cognitive impairments several years after long-term abstinence [2]. These decrements correlate with frequency and duration of methamphetamine use; however, we cannot rule out genetic or environmental factors which may contribute to individual differences in the duration and severity of these neurological and cognitive deficits.
Both acute and chronic stressors increase sensitivity to the development of a substance use disorder and increase the likelihood of relapse. Acutely, recovering methamphetamine addicts report higher craving ratings after given an acute stressor and individuals report relapsing to drugs of abuse during times of elevated stress [5] and [6]. Chronically, individuals in high-stress careers are more likely to develop alcohol dependence [7]. Chronic stress early in development also has long-lasting effects. Dube et al. [8] showed neglected or abused children have a nineteen times higher likelihood of developing a substance use disorder. In the clinical population, both acute and chronic stressors affect development of a substance use disorder and risk of relapse. Preclinical research suggests similar effects, in that after an acute stressor, animals self-administer more of a drug relative to those which did not receive a stressor [9], [10], and [11]. Chronic, unpredictable stressors additionally result in higher conditioned place preference scores for cocaine and morphine [12]. Acute and chronic stressors also lead to larger neuronal impairments after methamphetamine administration. Animal studies indicate chronic stressors administered in adult animals lead to enhanced methamphetamine-induced damage in the striatum [13].
Similar effects are observed in adolescence and adulthood when animals undergo stressors early in development. Maternal separation is an animal model of chronic early life stress resulting in long-term behavioral and physiological changes. Relative to controls, separated male rats show an increased response to opiates, cocaine, and amphetamine [14], [15], and [16]. Both male and female separated rats additionally show an elevated locomotor response to a 1.0 mg/kg dose of methamphetamine [17]. Maternally separated males also show enhanced rewarding effects of opiates, cocaine, and amphetamine [18], [19], [20], and [21]. Further, maternal separation may [22] or may not [23] and [24] enhance methamphetamine reward sensitivity, depending on the behavioral measure used. However, separated animals acquire cocaine and methamphetamine administration more quickly relative to controls [21] and [22]. These studies indicate that early life stress increases sensitivity to drugs of abuse, and early life stress likely contributes to the development of substance dependence.
Stress can lead to several negative effects on the development and maintenance of addiction as well as contribute to long-term neural changes associated with drug use. Stress in adolescence or adulthood can exacerbate methamphetamine-induced damage to striatal dopamine terminals. To date, no studies have examined whether early life stress has similar effects. Given that early life stress leads to long-term changes in neural circuits and behavior, it stands to reason that stress early in life may contribute to the chronic effects of methamphetamine in the brain. Furthermore, the majority of preclinical studies use only male rats. This is problematic considering women tend to escalate drug taking behavior more quickly and have higher rates of relapse relative to men [25]. This study set out to determine whether chronic early life stress affects methamphetamine-induced striatal damage in males and females.
MATERIAL AND METHODS
2.1 Breeding
A total of eighteen Long-Evans breeding rats (Harlan, Indianapolis, IN) were pair housed by same sex and allowed a one-week habituation to the facility before breeding. Breeding pairs were housed together in hanging wire cages and monitored for the presence of a vaginal plug for up to five days. Pairs were separated upon visualization of a vaginal plug and returned to plastic tub cages. Males were returned to their same sex cage mate and females were housed individually and monitored for pregnancy. Pregnant females remained untouched except for cage changes until birth of the litter. A total of 90 rats derived from 9 litters of 10–12 pups, were used in the study. Animals were pseudo-randomly distributed among treatment groups (n = 8–14 rats of each sex per group) in adulthood to control for litter effects.
2.2 Separation
Separation and control procedures began after birth. The day of birth was designated post natal day (PND) 0 and litters remained untouched until the next day. On PND 1, the pups were sexed and litters underwent pseudo-random assignment into control or separated groups to achieve roughly equal numbers for each group. During PND 2–14, control litters were removed from the dam and weighed, with this procedure lasting approximately fifteen minutes each day. Separated litters were removed from the dam, weighed, and individually separated in plastic containers for three hours per day during PND 2–14. Separated pups stayed on a heating pad, maintained at a constant temperature of 32°C, and were checked every 30 minutes for signs of impaired thermoregulation (i.e., visible changes in skin color or respiratory rate). After the three hour separation period, pups were returned to the dam. Identification of individual pups occurred by use of a non-toxic pen until PND 6–7, at which point ear punches were used for identification. Pups remained untouched, except for weekly cage changes, with the dam during PND 15–20. Weaning of pups occurred on PND 21, at which time pups were weighed and housed in same sex pairs. Starting on PND 40, pups were handled for about 5 minutes daily until the start of testing between PND 60–70.
2.3 Methamphetamine Dosing
(±)Methamphetamine hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile 0.9% saline at a concentration of 5.0 mg/ml (expressed as salt weight). Between PND 60–70 animals received saline or methamphetamine (METH) injections over an eight hour period. Core body temperature measurements were taken every two hours starting between 8:00am–8:30am. A thermocouple probe (Fluke, Wilmington, NC) was inserted approximately five to six millimeters into the rectum for five to ten seconds to obtain a stable baseline temperature. Rats then received one subcutaneous injection of 5.0 mg/kg METH or 0.9% saline (1.0 ml/kg) every two hours for a total of four injections. Rectal temperatures were taken one hour after every injection of saline or METH. If temperature exceeded 40°C, animals would have been cooled; however, no animals achieved this temperature during the course of the experiment. Room temperature was maintained between 22–23°C during all injections. After the final temperature measurement, rats were returned to their home cages and left undisturbed for seven days. After seven days, rats underwent euthanasia by anesthetic overdose of Somnasol (390 mg pentobarbital and 50 mg phenytoin, Henry Schein Animal Health, Dublin, OH). The brain was dissected out and the striatum was collected by use of a rodent brain matrix and a 0.3mm micropunch. Tissue samples were placed in Eppendorf tubes and immediately placed on dry ice, followed by storage at -80°C until quantification by Western Blot. Sacrifice seven days after METH administration was selected to determine the effects of MS on terminal damage as opposed to acute regulation of dopamine systems. METH acutely leads to the sequestration of DAT in the cytosol 24–72 hours after METH administration, but levels return to normal after this time period [26]. Taking brain tissue seven days after METH administration provides information on the effect of METH on dopamine markers after the acute effects are over.
2.4 SDS-Page Western Blotting
Tissue samples were thawed on ice and were homogenized in RIPA buffer using a rotor and pestle. Samples were centrifuged at 12,000g for 20 minutes. The supernantant was aliquoted into Eppendorf tubes and stored at −80°C. Each sample underwent a bicinchoninic acid protein concentration assay (Pierce, Rockford, IL) followed by western blot. Following heat denaturing, samples were loaded into 12% polyacrylamide gels with 10µg protein per lane. Samples were transferred to PVDF membranes and blocked for 3 hours in 5% milk in Tris-buffered saline (TBS). Membranes were washed 3 × 5 minutes and incubated in primary antibody for β-actin (raised in mouse;1:30,000; Proteintech, Chicago, IL), dopamine transporter (raised in goat; – DAT 1: 1,000; Santa Cruz, Santa Cruz, CA), and tyrosine hydroxylase (raised in mouse; – TH 1:1,000; EMD Millipore, Billerica, MA) in 5% milk in Tris-buffered saline with Tween 20 (TBST) overnight. Membranes were washed 3 × 10 minutes in TBST followed by IR-Dye secondary antibodies, anti-goat or anti-mouse (1:5,000; Li-Cor, Lincoln, NE) incubation in 5% milk in TBST for 1 hour. Membranes were washed 3 × 10 minutes in TBS and imaged by use of an Odyssey Clx imager (Li-Cor, Lincoln, NE). Fluorescent signals are directly proportional to the amount of the target protein. These values were normalized to β-actin and reported as proportion of control group (control-saline animals).
2.5 Data Analysis
Data were analyzed in SPSS. Since it has previously been demonstrated there are marked differences between males and females in responses to METH, separate ANOVAs were run for males and females. Rectal temperature data were analyzed using two mixed model ANOVAs with between subjects variables as condition (separated × control) and drug (saline × meth) and within subjects variable as time (baseline, 1 hour, 3 hours, 5 hours, 7 hours). Western blotting data were analyzed by use of the Odyssey imaging program. Fluorescent intensity for each band was normalized to β-actin and reported as proportion of control (control-saline males or control-saline females). Data were run through a 2×2 between-subjects ANOVA for each sex with factors as: condition (separated × control) and drug (saline × METH). Separate ANOVAs were run for dopamine transporter and tyrosine hydroxylase. Significance was set at α=0.05.
RESULTS
3.1 Rectal Temperatures
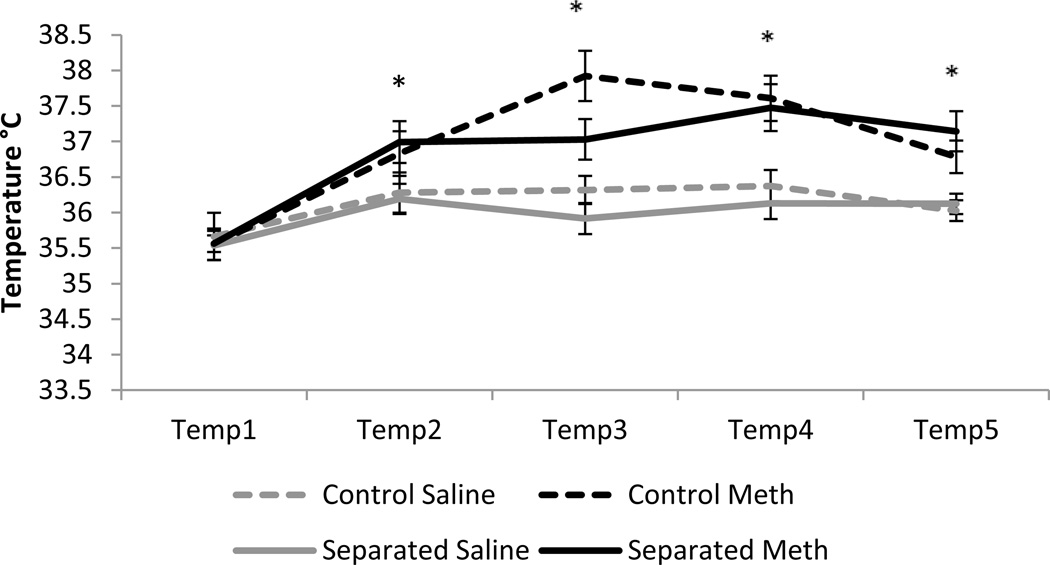
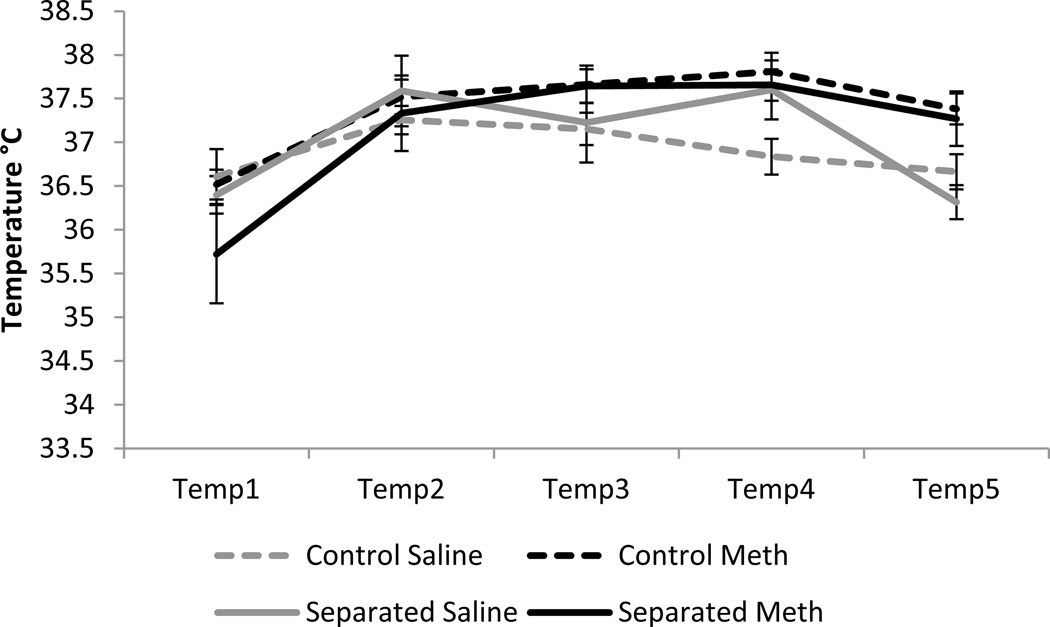
Two repeated-measures ANOVAs were run for males and females for temperature during drug administration. For males, there was a significant effect of time (F4,80 = 27.13, p<0.001), a significant time × drug interaction (F4,80 = 5.48, p<0.001) and no other significant within-subjects effects. There was a significant effect of drug (F1,83 = 15.09, p<0.001) with methamphetamine increasing rectal temperature. There were no significant effects of condition or a condition × drug interaction (figure 1). For females, there was a significant effect of time (F4,66 = 17.86, p<0.001) and no other significant within-subjects interactions. There were no significant between-subjects effects of condition or drug (figure 2).
Figure 1.
Rectal temperature in males across five two hour time points. Methamphetamine significantly increased rectal temperatures in both control and separated animals. * p<0.05, bars represent SEM.
Figure 2.
Rectal temperature in females across five two hour time points. Rectal temperatures did not differ as a result of condition or drug.
3.2 Western Blotting
For DAT levels, two separate 2 × 2 between-subjects ANOVAs were run for males and females, and the same was run for TH levels. For DAT in males, there was a significant effect of drug (F1,40 = 32.00, p<0.01), condition (F1,40 = 9.08, p<0.01), and condition × drug interaction (F1,40 = 8.4, p<0.01). A test of simple effects revealed that the effect of drug on DAT levels was significant for separated animals (F1,37 = 36.8, p<0.01) but not for control animals (F1,37 = 3.63, p>0.05) (figure 3). For females, there was no significant effect of drug, condition, or a drug × condition interaction (figure 4). For TH in males, there was a significant effect of drug (F1,44 = 7.18, p<0.05) with methamphetamine decreasing TH. There were no significant effects of condition or a drug × condition interaction (figure 5). For TH in females, there were no significant effects of drug, condition, or drug × condition interaction (figure 6).
Figure 3.
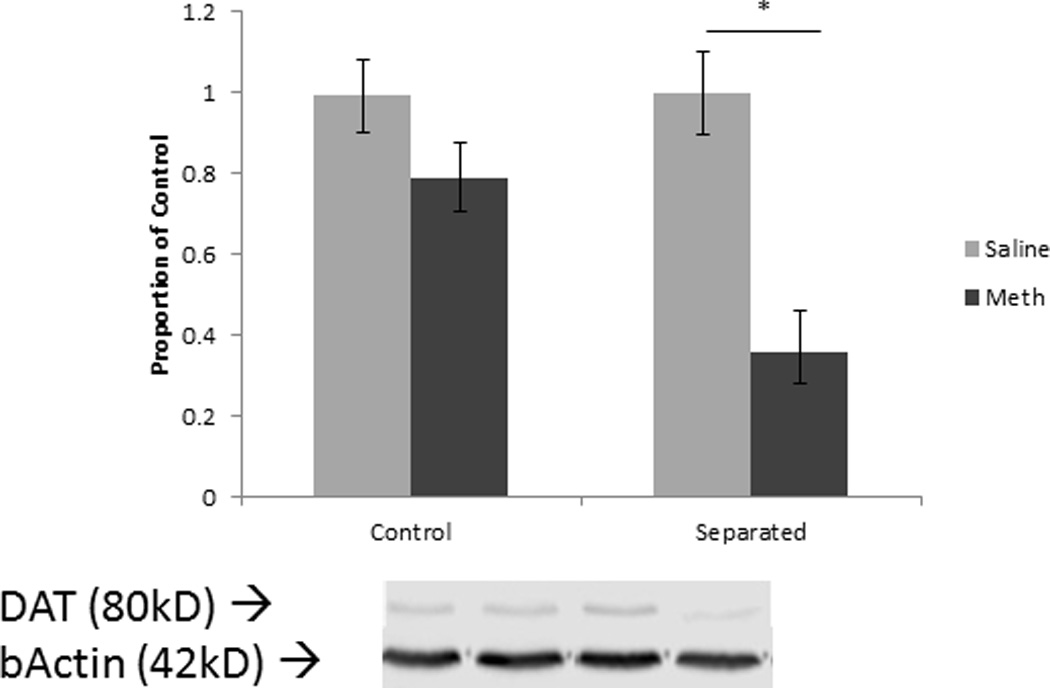
DAT levels in the striatum in males. * p<0.05; Error bars represent SEM.
Figure 4.
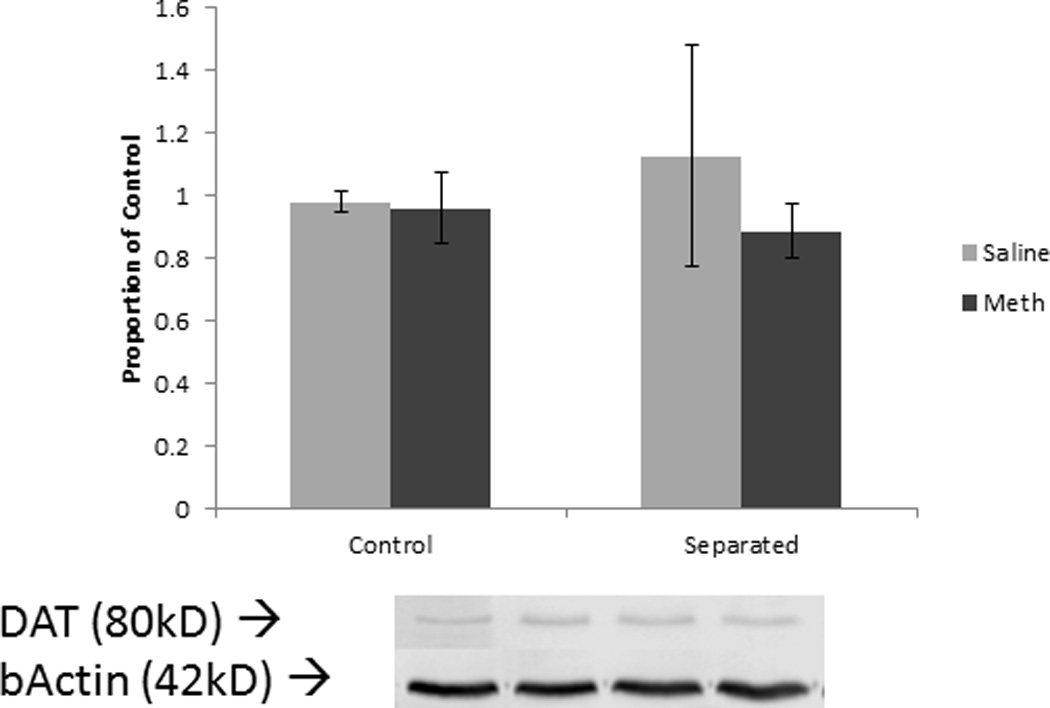
DAT levels in the striatum in females. Error bars represent SEM.
Figure 5.
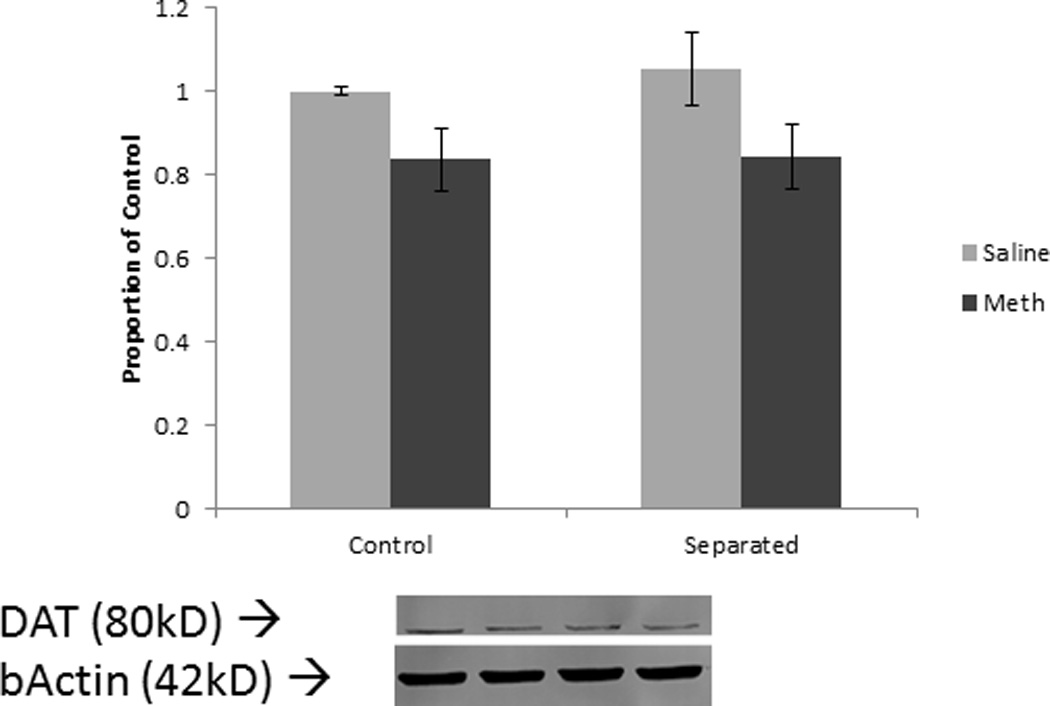
Tyrosine hydroxylase levels in the striatum in males. * p<0.05; Error bars represent SEM.
Figure 6.
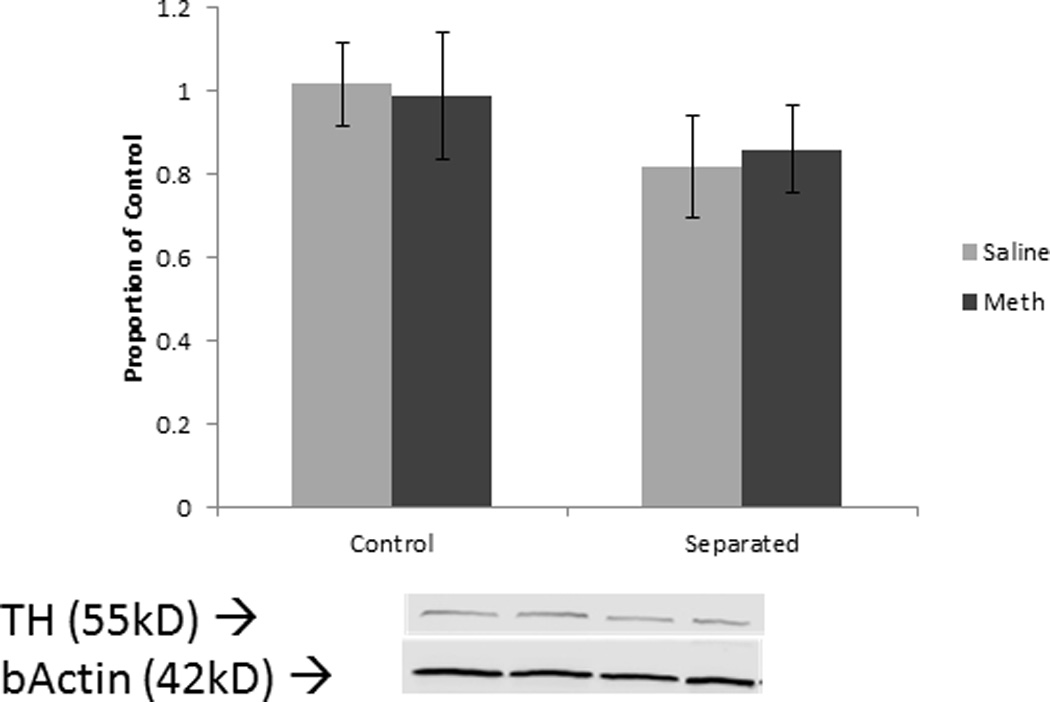
Tyrosine hydroxylase levels in the striatum in females. Error bars represent SEM.
4. DISCUSSION
Separated males exhibited the greatest reduction in dopamine transporters seven days after methamphetamine administration. Tissue was taken seven days after methamphetamine dosing, suggesting DAT reductions remain semi-stable and are not a result of acute down-regulation or sequestration after the methamphetamine binge. Both male groups exhibited a decrease in TH indicating an effect of methamphetamine. However, since DAT levels exhibited the largest decline in separated males, these animals may be more susceptible to the effects of methamphetamine striatal damage relative to control animals. In the current study, methamphetamine additionally elevated core body temperatures in both control and separated males to the same degree. Although core body temperatures did not differ, separated animals still exhibited a larger depletion in dopamine transporters. This suggests the effect is likely due to mechanisms other than impaired thermoregulation. However, since brain temperature was not measured directly, this cannot be ruled out completely.
Early life stress has many long lasting effects. Previous studies indicate early life stress alters several behavioral effects of psychostimulants including increased sensitivity to psychostimulant reward and locomotor activity [18], [21], and [22]. Although no direct studies exist to date, sensitivity to psychostimulant-induced locomotor activity likely predicts psychostimulant-induced monoaminergic damage. Neurotoxic dosing of amphetamines causes mice to become more sensitive to the locomotor activating effects of amphetamines, and the time course of this sensitization mirrors the time course of loss of striatal DA markers [27] and [28]. Since early life stress already increases sensitivity to the locomotor effects of psychostimulants, repeated doses of these drugs may have a potentiated neurotoxic effect on maternally separated male animals.
Differences in dopamine systems between separated and non-separated rats may underlie this effect. Methamphetamine reverses the direction of the dopamine transporter, leading to elevated extracellular dopamine. Excess dopamine leads to increased methamphetamine-induced striatal damage [29]. Neonatally isolated rats also exhibit increased dopamine release in the nucleus accumbens after an acute amphetamine injection [30]. Separated males also show decreased dopamine transporter binding in the nucleus accumbens core and caudate-putamen relative to controls [31]. Fewer transporters likely results in the accumulation of dopamine in the synaptic cleft, leading to increased damage by excess dopamine. However, other contributing mechanisms cannot be ruled out at this point in time. Overall, the data presented suggests early life stress potentiates methamphetamine-induced decreases in dopamine markers in males.
Unlike males, separation did not affect methamphetamine-induced reductions in DAT or TH levels in females. Many studies on maternal separation and psychostimulants only tested males [18], [21], and [22] or have tested females in adolescence [17], making it difficult to determine whether maternal separation affects females and males similarly. Studies of unstressed adult females given neurotoxic methamphetamine doses have found decreased markers of dopamine systems [32] and [33]. However, these studies used very high, single doses of methamphetamine (20.0 or 40.0 mg/kg) and reported males exhibited a greater decrease than females. As this study employed a lower dose relative to other studies, Western blotting may not have been sensitive enough to detect minor changes in DAT or TH levels. Autoradiography, receptor binding, or more sensitive assays may better detect slight but significant alterations in protein levels.
Contrary to some studies, the current study did not indicate methamphetamine-induced depletions in DAT or TH levels in females. Previous studies suggest females exhibit the greatest depletion in DAT levels three days after 40 mg/kg methamphetamine, but these levels tend to recover in females, but not males, seven days after a dose [32], [33], and [34]. Since the current study employed a time point of seven days after the final injection, females may have reached peak depletion and started to recover to baseline by the time they were sacrificed. Further, it is well documented that elevated body temperature contributes to increased methamphetamine damage. In the current study, methamphetamine did not significantly increase core body temperature in control or separated females. This lack of elevated core body temperature likely contributed to the divergent effects on DAT and TH expression in males and females.
To date, no clinical research exists on the effects of early life stress or chronic stress on methamphetamine-induced dopamine terminal damage. Human studies do, however, indicate several effects of stress on dopamine system functioning. Children who have been neglected or abused show lower fMRI activity in areas of the basal ganglia in response to natural rewards, indicating early life stress may alter neural response to various rewards [34] and [35]. Physiological measurements of plasma cortisol concentrations after a laboratory psychosocial stressor positively correlate with amphetamine-induced dopamine release in the striatum, suggesting acute effects of stress on amphetamine-induced dopamine release [36]. Additionally, adults with histories of childhood stress exhibit greater dopamine release in the striatum after an injection of amphetamine, indicating an enhanced response to drugs of abuse [37]. These studies suggest stress affects dopamine functioning and increases amphetamine-induced dopamine release. However, future studies will need to further evaluate the role of early life stress in methamphetamine-induced neural damage in human users.
The study is not without limitations. Tissue was only taken seven days after the final methamphetamine injection. Because a single time point was used, we cannot determine the duration of early life stress effects on dopamine systems in males, nor can we determine whether early life stress affects females in the short-term. Only DAT and TH markers were quantified. Although reductions were observed in these markers, several other aspects of the dopamine system or other neurotransmitter systems may have additionally been altered. Future studies should create a more comprehensive picture of methamphetamine-induced damages in separated animals. Finally, because females and males were analyzed separately, we cannot make a direct comparison between the sexes. Without measurements of plasma drug concentrations, we cannot rule out the possibility that the lack of neurotoxic effect in females was due to more extensive metabolism of METH or fluctuating hormonal levels. Future studies should take care to control for estrous cycle phase on methamphetamine-induced hyperthermia and striatal damage.
5. CONCLUSIONS
To our knowledge, this was the first study examining the effects of early life stress on methamphetamine-induced striatal dopamine damage. The results suggest early life stress may increase methamphetamine induced dopamine transporter loss in males, regardless of increased temperature, compared to controls. Additionally, neither control nor separated females exhibited a decrease in DAT or TH levels after methamphetamine, nor did they exhibit significantly elevated core body temperatures. This has implications for human research. Particularly, males exposed to childhood abuse or neglect have an increased likelihood of developing a substance abuse disorder relative to non-abused males and, based on the current results, may also have an increased likelihood of psychostimulant-induced damage to dopamine systems.
Highlights.
Early life stress increased methamphetamine-induced dopamine damage in the striatum
This effect occurred in male separated rats but not females
Core body temperatures were increased by methamphetamine in male rats only
There was no difference in core body temperature between separated and control males
Acknowledgements
The authors would like to acknowledge the National Center for Research Resources (5P20RR016464-11) and the National Institute of General Medical Sciences (8 P20 GM103440-11) for funding the project and for providing access to the Odyssey Imaging System. The authors also wish to thank Dr. Laurel Raftery, Kelly AbuAli, Matthew Eby, John Egan, Aisha Fowler, Ana Reyes, and the UNLV animal care staff for their help with the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration. Rockville, MD: 2013. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46. HHS Publication No. (SMA) 13-4795. [Google Scholar]
- 2.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. Journal of Neuroscience. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. American Journal of Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. American Journal of Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 5.King G, Alicata D, Cloak C, Chang L. Psychiatric symptoms and HPA axis function in adolescent methamphetamine users. Journal of Neuroimmune Pharmacology. 2010;5(4):582–591. doi: 10.1007/s11481-010-9206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Söderpalm A, Nikolayev L, de Wit H. Effects of stress on responses to methamphetamine in humans. Psychopharmacology (Berlin) 2003;170(2):188–199. doi: 10.1007/s00213-003-1536-5. [DOI] [PubMed] [Google Scholar]
- 7.Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. Journal of Studies on Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- 8.Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: The adverse childhood experiences study. Pediatrics. 2003;111(3):564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- 9.Kitanaka N, Kitanaka J, Takemura M. Behavioral sensitization and alteration in monoamine metabolism in mice after single versus repeated methamphetamine administration. European Journal of Pharmacology. 2003;474(1):63–70. doi: 10.1016/s0014-2999(03)02015-6. [DOI] [PubMed] [Google Scholar]
- 10.Maccari S, Piazza PV, Deminiere JM, Lemaire V, Mormede P, Simon H, et al. Life events-induced decrease of corticosteroid type I receptors is associated with reduced corticosterone feedback and enhanced vulnerability to amphetamine self- administration. Brain Research. 1991;547:7–12. doi: 10.1016/0006-8993(91)90568-g. [DOI] [PubMed] [Google Scholar]
- 11.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction (Abingdon, England) 2001;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 12.Will MJ, Watkins LR, Maier SF. Uncontrollable stress potentiates morphine’s rewarding properties. Pharmacology Biochemistry and Behavior. 1998;60:655–664. doi: 10.1016/s0091-3057(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 13.Matuszewich L, Yamamoto BK. Chronic stress augments the long-term and acute effects of methamphetamine. Neuroscience. 2004;124(3):637–646. doi: 10.1016/j.neuroscience.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Kikusui T, Isaka Y, Mori Y. Early weaning deprives mouse pups of maternal care and decreases their maternal behavior in adulthood. Behavioural Brain Research. 2005;162(2):200–206. doi: 10.1016/j.bbr.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Matthews K, Hall FS, Wilkinson LS, Robbins TW. Retarded acquisition and reduced expression of conditioned locomotor activity in adult rats following repeated early maternal separation: Effects of prefeeding, d-amphetamine, dopamine antagonists and clonidine. Psychopharmacology. 1996;126(1):75–84. doi: 10.1007/BF02246414. [DOI] [PubMed] [Google Scholar]
- 16.Vazquez V, Giros B, Dauge V. Maternal deprivation specifically enhances vulnerability to opiate dependence. Behavioral Pharmacology. 2006;17(8):715–724. doi: 10.1097/FBP.0b013e3280116e6f. [DOI] [PubMed] [Google Scholar]
- 17.Pritchard LM, Hensleigh E, Lynch S. Altered locomotor and stereotyped responses to acute methamphetamine in adolescent, maternally separated rats. Psychopharmacology. 2012;223(1):27–35. doi: 10.1007/s00213-012-2679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Der-Avakian A, Markou A. Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience. 2010;170(4):1189–1198. doi: 10.1016/j.neuroscience.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: evidence for opiate mediation of social affect. Pharmacology and Biochemical Behavior. 1978;9(2):213–220. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- 20.Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Research. 2000;875(1–2):44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- 21.Moffett MC, Harley J, Francis D, Sanghani SP, Davis WI, Kuhar MJ. Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. The Journal of Pharmacology and Experimental Therapeutics. 2006;317(3):1210–1218. doi: 10.1124/jpet.106.101139. [DOI] [PubMed] [Google Scholar]
- 22.Lewis CR, Staudinger K, Schedk L, Olive MF. The effects of maternal separation on adult methamphetamine self-administration, extinction, reinstatement, and MeCP2 immunoreactivity in the nucleus accumbens. Frontiers in Psychiatry. 2013;55(4) doi: 10.3389/fpsyt.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faure J, Stein DJ, Daniels W. Maternal separation fails to render animals more susceptible to methamphetamine-induced conditioned place preference. Metabolic Brain Disease. 2009;24(4):541–559. doi: 10.1007/s11011-009-9158-1. [DOI] [PubMed] [Google Scholar]
- 24.Hensleigh E, Pritchard LM. The effect of early environmental manipulation on locomotor sensitivity and methamphetamine conditioned place preference reward. Behavioral Brain Research. 2014;268:66–71. doi: 10.1016/j.bbr.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker JB, Hu M. Sex differences in drug abuse. Frontiers in Neuroendocrinology. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. European Journal of Pharmacology. 2003;479(1–3):153–158. doi: 10.1016/j.ejphar.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 27.Itzhak Y, Achat-Mendes CN, Ali SF, Anderson KL. Long-lasting behavioral sensitization to psychostimulants following p-chloroamphetamine-induced neurotoxicity in mice. Neuropharmacology. 2004;46(1):74–84. doi: 10.1016/s0028-3908(03)00316-2. [DOI] [PubMed] [Google Scholar]
- 28.McMillen BA, Scott SM, Williams HL. Effects of subchronic amphetamine or amofonelic acid on rat brain dopaminergic and serotonergic function. Journal of Neural Transmission Genetic Section. 1991;83(1–2):55–66. doi: 10.1007/BF01244452. [DOI] [PubMed] [Google Scholar]
- 29.Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. Journal of Neurochemistry. 2008;105(3):605–616. doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kehoe P, Shoemaker WJ, Triano L, Hoffman J, Arons C. Repeated isolation in the neonatal rat produces alterations in behavior and ventral striatal dopamine release in the juvenile after amphetamine challenge. Behavior Neuroscience. 1996;110(6):1435–1444. doi: 10.1037//0735-7044.110.6.1435. [DOI] [PubMed] [Google Scholar]
- 31.Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. European Journal of Neuroscience. 2004;19(7):1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- 32.Bourque M, Dluzen D, Di Paolo T. Sex and temporally-dependent effects of methamphetamine toxicity on dopamine markers and signaling pathways. Neuropharmacology. 2012;62:2363–2372. doi: 10.1016/j.neuropharm.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Dluzen D, McDermott JL, Darvesh AS. Relationship among gender, age, time, and temperature in methamphetamine-induced striatal dopaminergic neurotxicity. Neuroscience. 2010;167(4):985–993. doi: 10.1016/j.neuroscience.2010.02.076. [DOI] [PubMed] [Google Scholar]
- 34.Bourque M, Liu B, Dluzen D, Di Paolo T. Sex differences in methamphetamine toxicity in mice: Effect on brain dopamine signaling pathways. Psychoneuroendrocrinology. 2010;36:955–969. doi: 10.1016/j.psyneuen.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagali DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta MA. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation in life. Journal of Cognitive Neuroscience. 2010;22:2316. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- 37.Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, et al. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropyschopharmacology. 2007;32(11):2310–2320. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- 38.Oswald LM, Wand GS, Kuwabana H, Wong DF, Zhu S, Brasic JR. History of childhood adversity in positively associated with ventral striatal dopamine responses to amphetamine. Psychopharmacology. 2014;231(12):2417–2433. doi: 10.1007/s00213-013-3407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]