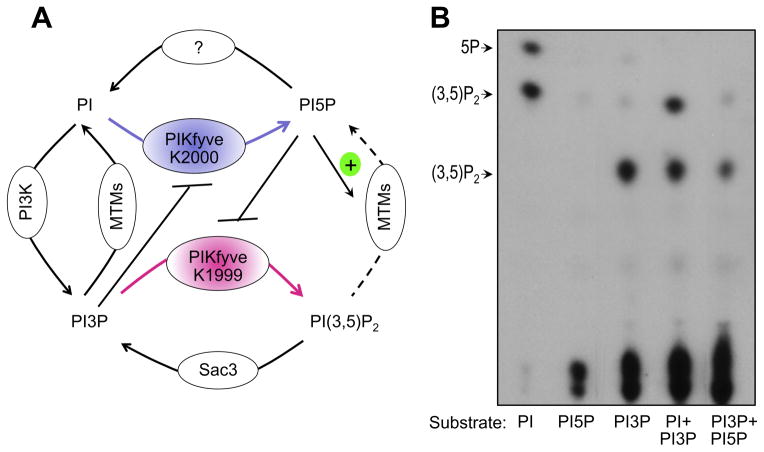
Fig. 3.
Proposed model for PIKfyve dual activity and regulation in vivo. (A) The differential role of K1999 and K2000 residues from the predicted PIKfyve substrate binding pocket in production of PtdIns(3,5P)2 and PtdIns5P, respectively, has been established both in vivo and in vitro [34]. Feed-forward inhibition of PIKfyve-catalyzed PtdIns5P production from PtdIns in the presence of high levels of PtdIns3P was observed previously in vitro [9] and is shown in B. Feedback inhibition of PIKfyve-catalyzed PtdIns(3,5)P2 synthesis from PtdIns3P by high levels of PtdIns5P is suggested based on reduced in vitro synthesis of PtdIns(3,5)P2 in the presence of PtdIns5P. (Sbrissa D & Shisheva A, unpublished observation that is now presented in B). Activation of MTM, MTMR3 and MTM6 by PtdIns5P was observed in vitro [55]. See text for more detail. (B) PIKfyve lipid kinase activity in vitro was determined with the indicated substrates alone or in various combinations, added at a molar ration 1:1, under conditions, specified in Fig. 2A. Presented is the TLC autoradiogram of generated products whose identity was confirmed by HPLC analyses.