Fig. 7.
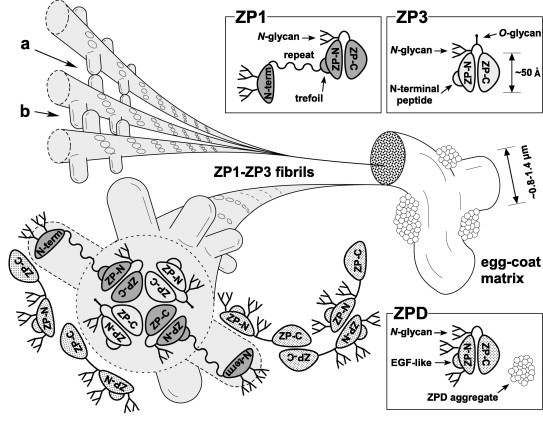
A tentative model for the architecture of the chicken egg-coat matrix. A schematic diagram of the chicken egg-coat matrix architecture was drawn based on the results from the present and previous studies. Chicken ZP1 containing the N-terminal (N-term), repeat, trefoil, ZP-N and ZP-C domains, and N-glycans on both the N-terminal domain and the ZP-N/ZP-C linker region assembles with chicken ZP3 containing the N-terminal peptide, ZP-N and ZP-C domains, and N- and O-glycans on the linker region through the interactions between their ZP-C domains to form the helical ZP1–ZP3 fibrils. The N-terminal, repeat, trefoil domains of ZP1, the N-terminal peptide of ZP3 and above-mentioned glycans are exposed on the surface of the ZP1–ZP3 fibrils to overlie the ZP-N domains. The ZP1–ZP3 fibrils assemble into bundles through both intermolecular disulfide bonds between the N-terminal domains of ZP1 (a), and non-covalent interactions between the repeat domains of ZP1 (b), to form the fibrous ZP1–ZP3 matrix. In addition, chicken ZPD containing the calcium-binding EGF-like (EGF-like), ZP-N and ZP-C domains, and N-glycans on both the ZP-N domain and the ZP-N/ZP-C linker region forms homopolymers in the head-to-head and tail-to-tail arrangements and attaches to the ZP1–ZP3 fibrils located on the surface of the interstices of ZP1–ZP3 matrix to form aggregates. Schematic diagrams of ZP1, ZP3 and ZPD are drawn based on the domain structure of chicken ZP1 and the crystal structure of full-length chicken ZP3 (PDB accession code: 3NK3) [16]. All the putative N-glycosylation sites of the ZP glycoproteins are occupied in this scheme.
