Fig. (5).
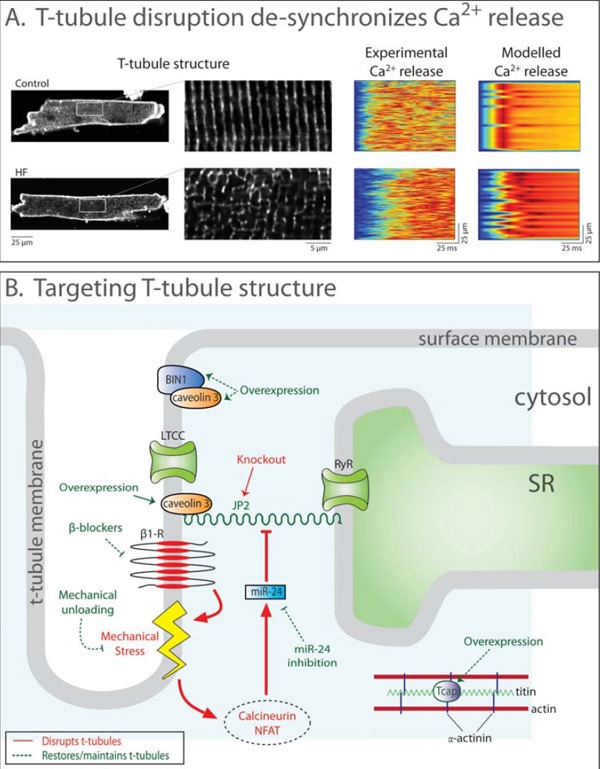
T-tubule disruption in heart failure and pathways to restore t-tubule structure. A: Confocal images post-infarction murine cardiomyocytes stained with di-8-ANEPPS show disrupted t-tubule structure in heart failure (left panel, magnified in insets). Accordingly, SR Ca2+ release was desynchronized in comparison with sham-operated controls. Mathematical modeling quantitatively reproduced the dyssynchronous pattern of Ca2+ release when changes in t-tubule organization, RyR threshold, and SR Ca2+ content were accounted for (right panels). Data are adapted from [193], with permission. B: Ttubule integrity and function is dependent on several proteins. JP2, along with caveolin 3, anchors t-tubules to the SR membrane. Increased calcineurin-NFAT signaling resulting from mechanical stress downregulates JP2 and disrupts t-tubules. JP2 expression may be restored by mechanical unloading, blockade of calcineurin-NFAT signaling, or by overexpressing the stretch-sensitive protein Tcap. BIN1 is involved in t-tubule growth and is downregulated in heart failure. Overexpression of BIN1 may therefore attenuate t-tubule loss.
