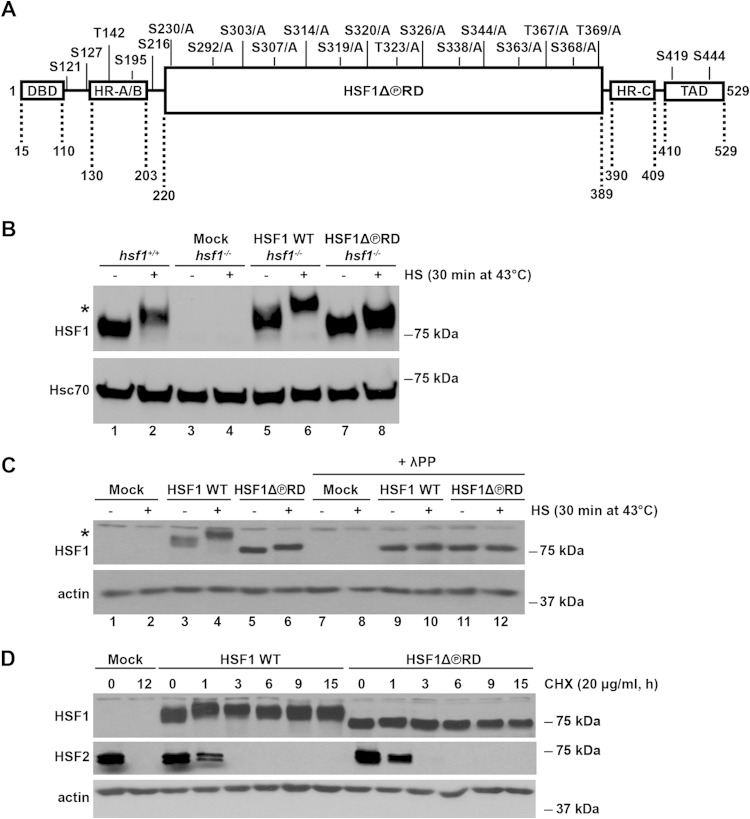
FIG 1.
Characterization of the HSF1 mutant that is phosphorylation-deficient within the RD, HSF1Δ∼PRD. (A) Schematic illustration of the HSF1 functional domains with the known phosphorylation sites. In HSF1Δ∼PRD, 15 phosphorylation sites in the regulatory domain (RD) were mutated from serine (S) and threonine (T) residues to alanines (A) as indicated. Additional HSF1 domains include the DNA-binding domain (DBD), heptad repeat domains (HR-A/B and HR-C), and transactivation domain (TAD). Note that the figure is not drawn to scale. (B) hsf1−/− MEFs were transfected with Mock plasmid [pcDNA3.1/myc-His(−)A], Myc-His-HSF1 WT, or Myc-His-HSF1Δ∼PRD. hsf1+/+ represents the endogenous levels of HSF1 in MEFs. Cells were either left untreated (−) or exposed to heat shock (+). HSF1 protein levels from cell lysates were detected by Western blotting with anti-HSF1 antibody. Hsc70 is shown as a loading control. An asterisk indicates an HSF1 protein that migrates slower on SDS-PAGE due to hyperphosphorylation (53). The difference in size between the endogenous HSF1 from hsf1+/+ MEFs and exogenous HSF1 WT is caused by the Myc-His tag on the human HSF1 WT construct. (C) hsf1−/− MEFs were transfected as in panel B. Cells were either left untreated (−) or heat shocked (+). Cell lysates were treated with lambda protein phosphatase (+λPP) or left untreated. Samples were analyzed by using Western blotting. α-Actin is shown as a loading control. An asterisk indicates the HSF1 protein that migrates slower on SDS-PAGE due to hyperphosphorylation (53). (D) hsf1−/− MEFs were transfected as in panel B and treated with cycloheximide (CHX) at 37°C for the indicated times. Cell lysates were analyzed with anti-HSF1 and anti-HSF2 antibodies. α-Actin is shown as a loading control.