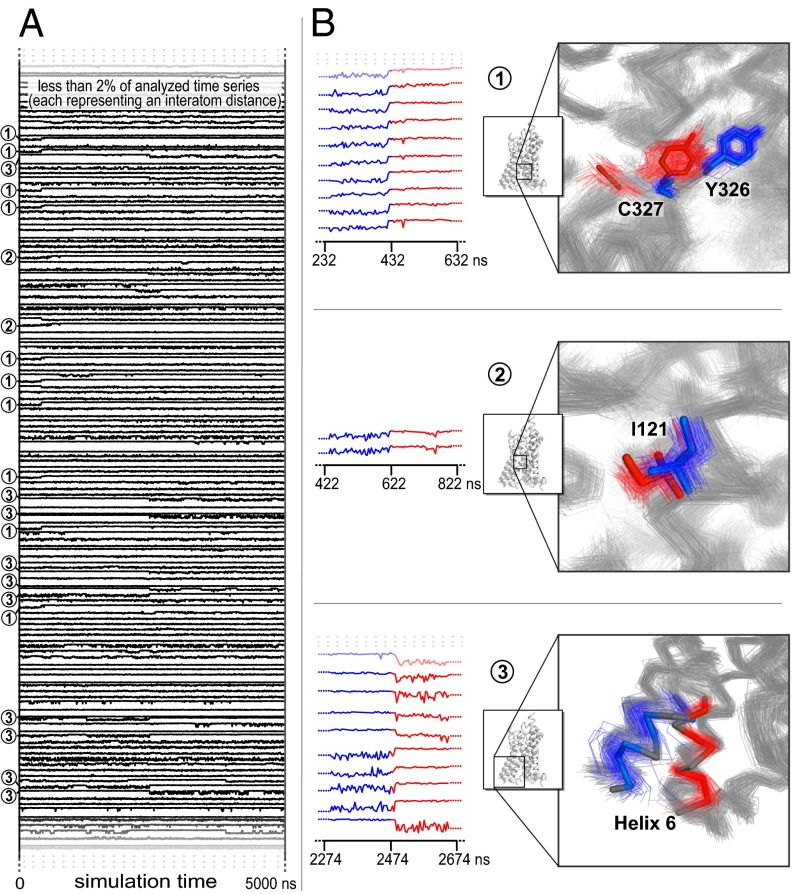
Fig. 3.
Application of SIMPLE to a simulation of the β2-adrenergic receptor transitioning spontaneously from its active state to its inactive state. (A) We used as inputs ∼14,400 observables across ∼1,000 time points. Each observable represented the distance between one pair of atoms, after application of a nonlinear transformation that emphasizes formation and breakage of direct contacts between atoms. (B) Three of the four conformational changes detected at a sensitivity parameter setting of λ = 1,300. Multiple simulation snapshots before and after each change are superimposed. The residues found to be involved in each change are shown in blue before each change and in red afterward. The remainder of the protein is shown in gray both before and after the change. The conformational changes illustrated are detected as simultaneous changes of 17, 2, and 122 input observables, respectively. The only detected change that is not displayed, a motion of residue Tyr219 and nearby residues, takes place about 50 ns before the third change displayed and is detected as a simultaneous change of 65 input observables. The detected conformational changes correspond closely to those identified by Dror et al. (31) through painstaking manual and visual analysis.