Fig. 2.
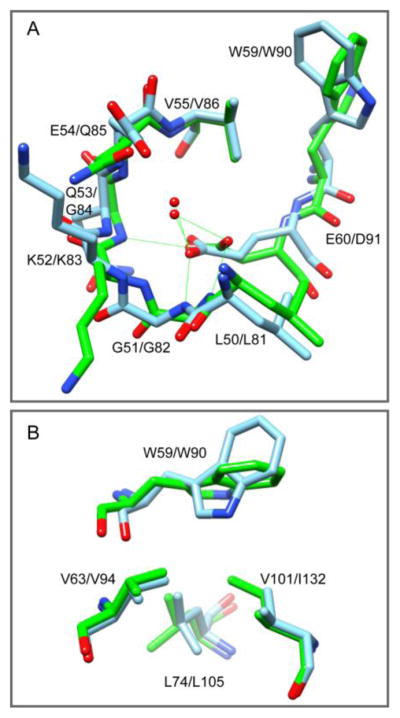
Superposition of the 0.96 Å resolution crystal structure for the first FKBP domain of FKBP51 (green) onto the G89P (blue) crystal structure. (A) The positioning of the 50’s loop backbone and the interactions of the Glu 60 / Asp 91 carboxylate groups are similar in the structures of the G89P variant [24] and the FK1 domain of FKBP51 [37]. However, the backbone segment connecting to the indole sidechain is shifted further away by ~1Å in the G89P crystal structure. (B) Underneath the indole ring of Trp 59, the Cδ1 of Ile 132 in FKBP51 projects into where a cavity is formed by the sidechains of Val 63, Leu 74 and Val 101 in the FKBP12 structure.
