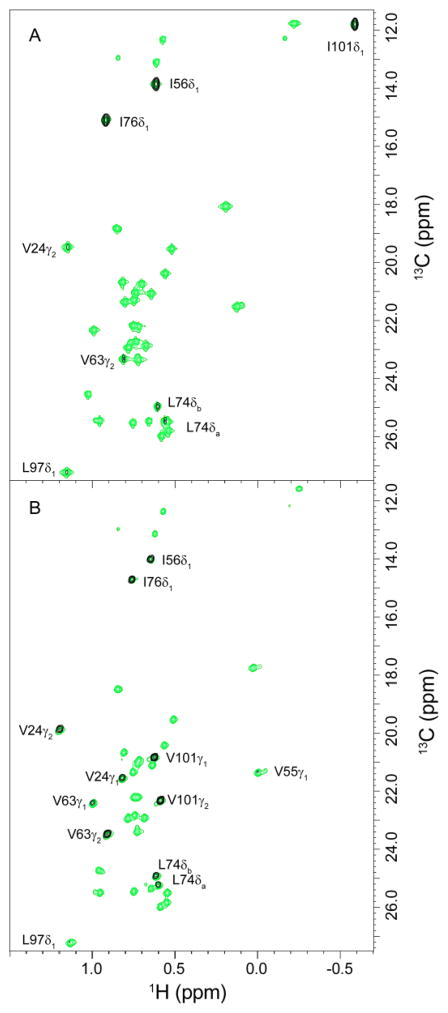
Fig. 3.
NOESY crosspeaks between the active site Trp 59 HNε1 and nearby methyl groups in the V101I variant (A) and wild type FKBP12 (B) for the F2-F3 plane at the indole 1HNε1 frequency from a 3D F1-filtered 1H-13C-1H NOESY spectrum of collected using a 200 ms mix time (black) is superimposed upon the reference 2D 1H,13C HSQC spectrum containing all selectively enriched valine Cγ, leucine Cδ and isoleucine Cδ positions (green). The observable crosspeaks from the 3D NOESY spectrum of the V101I variant predominantly reflect intensities that are consistent with the canonical orientation of the indole ring. The crosspeak for Val 24 Cγ2 is 10-fold weaker in the V101I spectrum. This crosspeak would be unobservable for the canonical orientation of the indole ring and is consistent with a corresponding 10-fold decrease in the population of the perpendicular reoriented indole ring, relative to the wild type protein.