Figure 1. Structural alignments of globins.
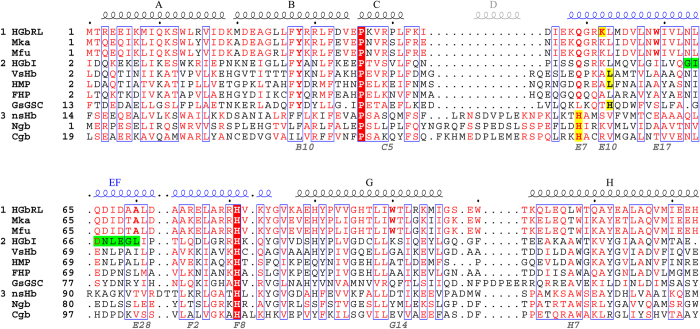
HGbRL is unique to the genus Methylacidiphilum (Group 1), presently found only in two other bacteria, M. kamchatkense (Mka) and M. fumariolicum (Mfu) (aligned by protein sequences). Its globin domain is closely related to bacterial globins (Group 2) with conserved Tyr(B10) and Gln(E7), as well as lacking helix D that is present in eukaryotic globins (Group 3). Thus far N-HGbRL is the only globin containing fused helices E and F (EF) among all the known structures, with the corresponding loop EF of HGbI (green) straightened into part of the elongated helix. Haem hexacoordination in eukaryotic globins invariably involves His(E7) (yellow), as seen in nonsymbiotic plant haemoglobin (nsHb), neuroglobin (Ngb) and cytoglobin (Cgb). In bacterial globins, on the other hand, hexacoordination involves residues at either position E10 or E11, such as Lys52(E10) in N-HGbRL, His66(E11) in GsGSC, and Leu57(E11) in both VsHb and HMP for haem shielding. Following the conventional numbering for the distal Gln/His(E7) and proximal His(F8), the fused helices E and F are numbered E6–E31 followed by F1–F11.