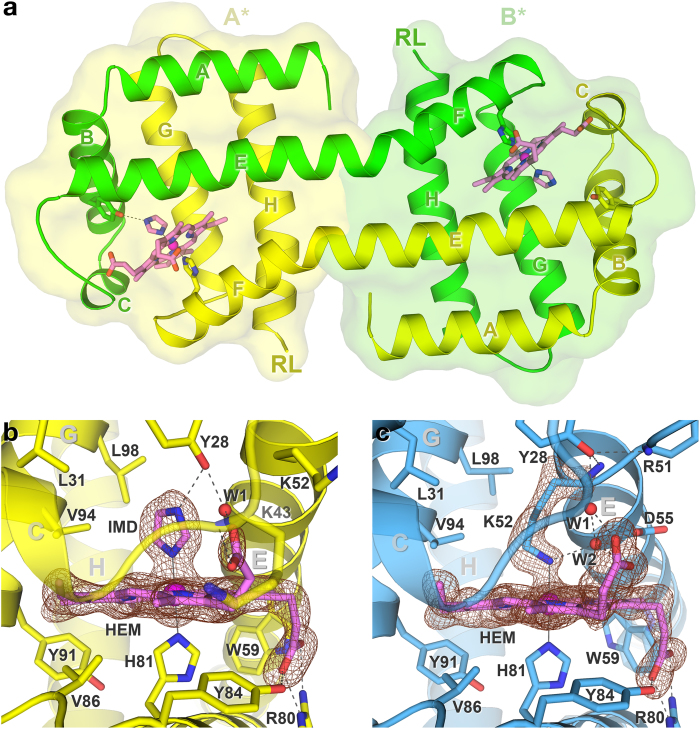
Figure 3. Structures of N-HGbRL in the open and closed forms.
(a) Site-swapped dimer of N-HGbRL. Both helices E and F unexpectedly fuse into an elongated helix, resulting in a globin fold, denoted A*, being made up of helices A–E from one subunit (green) and F–H from the other (yellow). While the two C-terminal Roadblock/LC7 (RL) domains are positioned at the opposite ends, they may still lie on the same side to interact with each other. (b) Open form of N-HGbRL. An imidazole (IMD) molecule binds to the haem (HEM) and the conserved Tyr28(B10). (c) Closed form of N-HGbRL. Helix E moves inwards and unravels its first helical turn for Lys52(E10) to bind to the haem, forming a unique Lys–His hexacoordinated structure. The Fo − Fc omit maps for both structures are contoured at 3 σ.