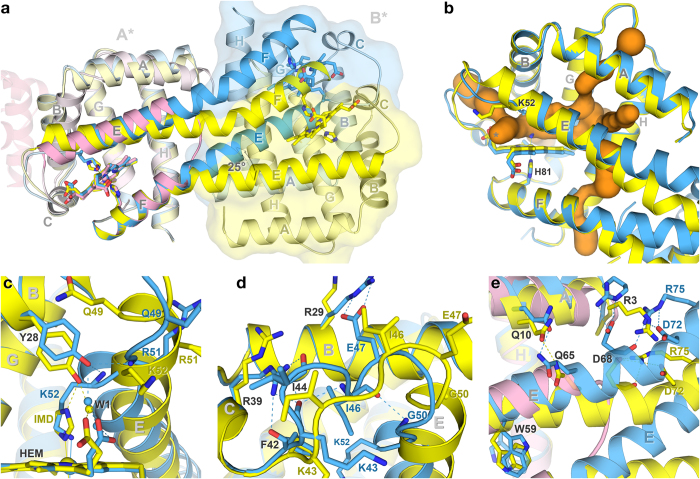
Figure 4. Conformational changes between the open and closed forms.
(a) Superposition of open (yellow) and closed (blue) forms of subunits A* of N-HGbRL with HGbI (pink) at helices F–H. A bending of ~25° of the fused helices E and F leads to a large shift in subunits B* of the two N-HGbRL forms. Dimerization of HGbI involves helices B of the first and the second (transparent) subunits. (b) Tunnels and distal pocket of N-HGbRL. Besides connecting to several tunnels (orange), the pocket in the distal site of the open form is also directly exposed to the solvent through an opening (*). (c) Conformational changes in distal site. Helix E moves outwards in the open form to allow Tyr28(B10) to bind to imidazole, whereas it moves towards the haem in the closed form and drives Tyr28(B10) away from the distal site. (d) Conformational changes in loop CE. While folding compactly into the distal site in the closed form, loop CE as well as helix E in the open form extend outwards to open up the distal site. (e) Conformational changes along the fused helices E and F between the two forms.