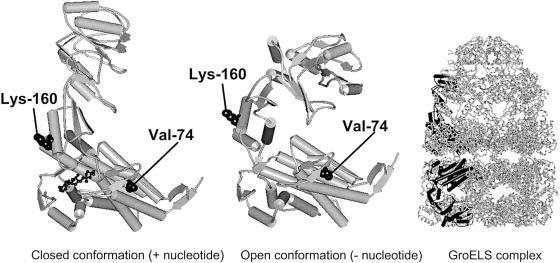
Figure 2.
Schematic representation of the structure of the Hsp60 chaperonin homologue GroEL from E. coli (Protein Data Bank entry 1AON). The architecture of a subunit in the closed and open conformations and an overview of the GroEL/GroES chaperonin complex with one subunit in the closed (upper ring) and open (lower ring) conformations highlighted in black are shown. The side chains of valine-74 and lysine-160, which correspond to valine-72 and asparagine-158, respectively, in human Hsp60, are shown in space-filling representation. The figure was produced with WebLab ViewerLite software (Molecular Simulations).