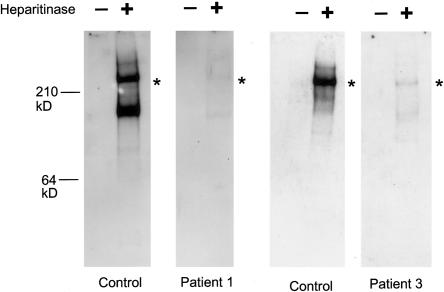
Figure 3.
Perlecan secreted by cultured fibroblasts in patients 1 and 3. Media from cultured normal or patient fibroblasts were concentrated, were then adjusted to contain 0.35g CsCl/g of 4M guanidine, and were centrifuged for 72 h at 40,000 RPM in a 50-Ti rotor at 12°C. A proteoglycan-containing fraction was pooled, concentrated, and digested with 0.1 mU heparitinase and 0.25 mU chondroitinase ABC (Seikagaku). Digested and undigested samples were electrophoresed in 3%–8% polyacrylamide tris-acetate gels, were transferred to nitrocellulose, and were blotted with polyclonal antibodies to the perlecan protein core. In patient 1, a perlecan protein core of ∼400 kD was detected after heparitinase digestion, but the amount was reduced significantly, correlating with the reduced amount of the wild-type perlecan transcript. Heparitinase treatment is necessary for the blotting, because perlecan-containing heparan sulfate chains do not transfer well onto a membrane because of the negatively charged heparan sulfate chains. As with patient 1, patient 3 fibroblasts secreted the 400-kD perlecan core protein in much more reduced amounts than did the control cells. The truncated perlecan in patient 3 is missing only ∼35–64 amino acids of the most C-terminal part of domain V, suggesting the mutant perlecan to be almost the same size as the wild-type perlecan. Asterisks (*) indicate positions of the ∼400-kD protein core.