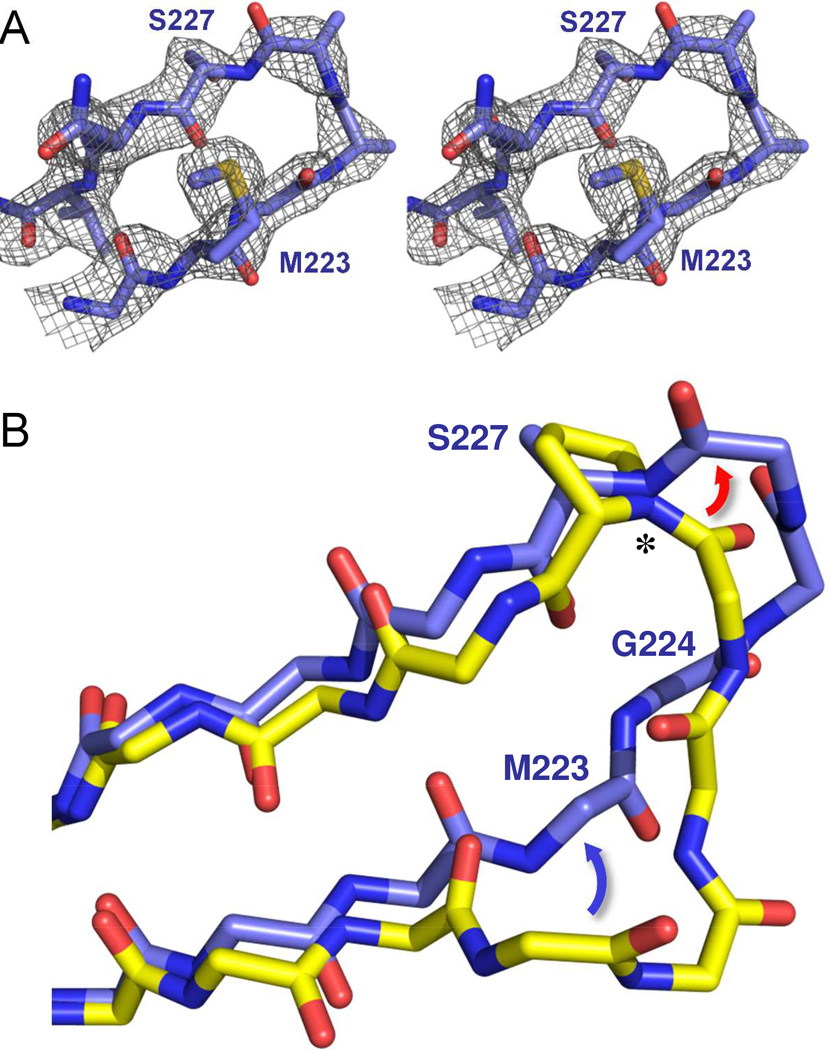
Figure 6. Structure of the β5-β6 loop in OXA-160.
(A) An Fo-Fc omit map of the β5-β6 loop (residues 222–229) contoured at 3.0 σ (PDB 4X53). (B) Effect of the P→S mutation on the trajectory of the β5-β6 loop. OXA-24/40 K84D/doripenem (3PAE, yellow) was aligned with OXA-160 V130D/aztreonam (blue; PDB 4X53) as described in Figure 4. Only the main-chain atoms are shown for clarity, with the exception of P/S227. The largest difference between OXA-24/40 and OXA-160 is in the region of G222-T226, with the area of bridge residue M223 moving away from the active site (blue arrow) and the region of T226 extending further from the surface of the enzyme (red arrow) to form a turn. The deviation is accompanied by a large change in the φ torsion angle (*) for residue 227.