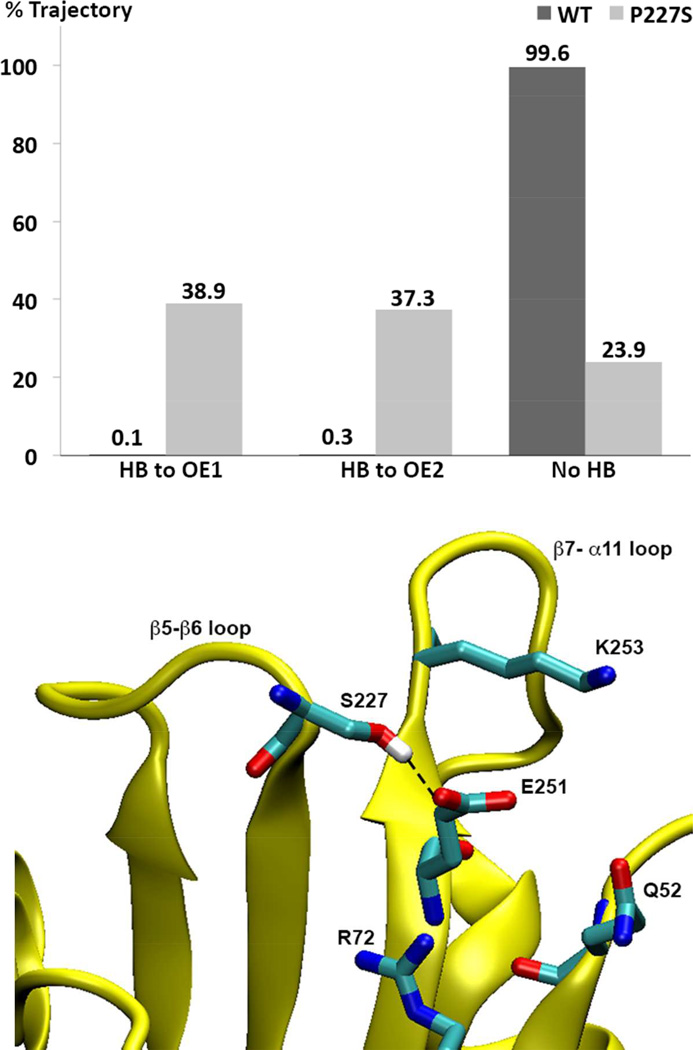
Figure 8. Prevalence of hydrogen bonding between S227 and E251 in OXA-160 and OXA-24/40.
Upper panel: For OXA-160 (OXA-24/40 P227S), the hydroxyl oxygen atom of S227 remains within 3.2 Å distance from E251 Oε1 and Oε2 atoms for 38.9% and 37.3% of the time, respectively. In contrast, the corresponding atoms in P227 (Cγ) and E251 in the WT enzyme remain greater than 3.2 Å apart 99.6% of the time. Hydrogen bonding was determined over the 38 ns after equilibration based on the following geometric criteria: D-A < 3.2 Å, H-A < 2.2 Å and DHA > 120°. When hydrogen bonded, the average D-A distance in OXA-160 is 2.7 Å (with an entire trajectory average of 3.8 Å). Lower Panel: A trajectory snapshot of a conformation that displays a typical hydrogen bond between S227 and E251.