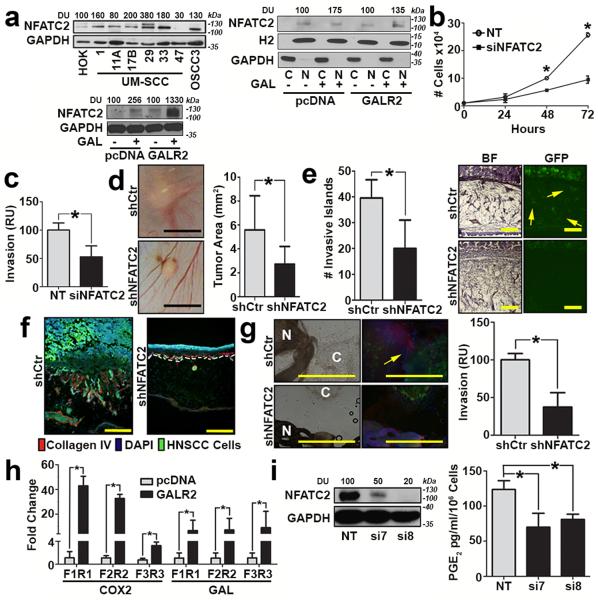
Figure 5. GALR2 promotes tumour progression and PNI via NFATC2.
(a, left upper panel) Multiple HNSCC cell lines express more NFATC2 than primary human oral keratinocytes (HOK); (a, left lower panel) UM-SCC-1-GALR2 and UM-SCC-1-pcDNA cells were induced with 5nM GAL for 2min and whole cell lysates were immunoblotted with anti-NFATC2. GAPDH was used as a loading control; (a, right panel) GAL (5nM) induces nuclear translocation of NFATC2 in UM-SCC-1-GALR2 and UMSCC-1-pcDNA cells. H2 (histone 2), and GAPDH were used as loading controls for nuclear and cytoplasmic proteins, respectively. (b and c) UM-SCC-1-GALR2 cells transfected with siNFATC2, show reduced proliferation (b) and invasion (c) compared to the same cells transfected with NT siRNA. In CAM, UM-SCC-1-GALR2 cells with constitutive knockdown of NFATC2 (shNFATC2) induce tumours that are (d) smaller, (scale bar = 5mm), (e) less invasive (arrows show invasive islands, scale bar = 200μm; *P<0.02, two sample t-test), and (f) less disruptive to the basement membrane (labelled with Collagen IV, scale bar = 200μm) than the same cells with control scrambled shRNA (shCtr). (g) UM-SCC-1-GALR2-shNFATC2 cells exhibit less PNI than controls (cancer cells labelled “C” fluoresce green and dorsal root ganglia (DRG) are labelled “N”; arrow identifies neurite growth, scale bar = 1mm). Graph shows quantification. (h) NFATC2 binds more to the promoter regions of PTGS2 (COX2) and GAL (GAL) in UM-SCC-1-GALR2 cells compared to control pcDNA. (i) When NFATC2 is downregulated with siRNA in UM-SCC-1-GALR2 cells, PGE2 secretion decreases. For CAM experiments, n = 6 for both; in vitro and DRG explant data are representative of three independent experiments each with three replicates. (*P < 0.05, two sample t-test; data represent mean + SD).