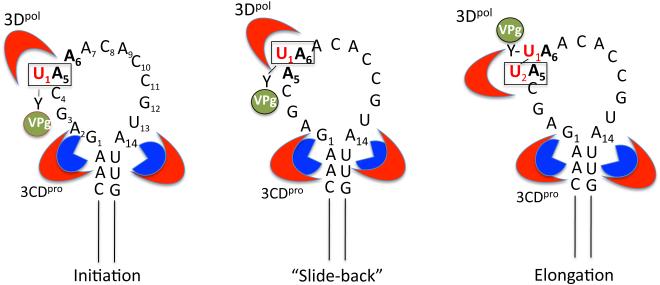
Fig. 5.
“Slide-back” model for VPgpUpU synthesis during initiation of RNA synthesis. The upper stem of cre(2C) interacts with 2 molecules of 3CDpro (or 3Cpro) (section 3.7). 3CDpro binds VPg with the back side of its 3Dpol domain, where another molecule of 3Dpol links UMP to the hydroxyl group of tyrosine in VPg. A5 in the loop of cre(2C) is the template for the linkage of the first UMP (U1) to VPg yielding VPgpU1 . VPgpU1 “slides-back” to hydrogen bond with A6 and the second UMP (U2) is templated again by the A5 nucleotide during the elongation step yielding VPgpU1U2. The nucleotides involved in the “slide back” are boxed. Nucleotides A5 and A6 of the conserved motif are shown in bold.