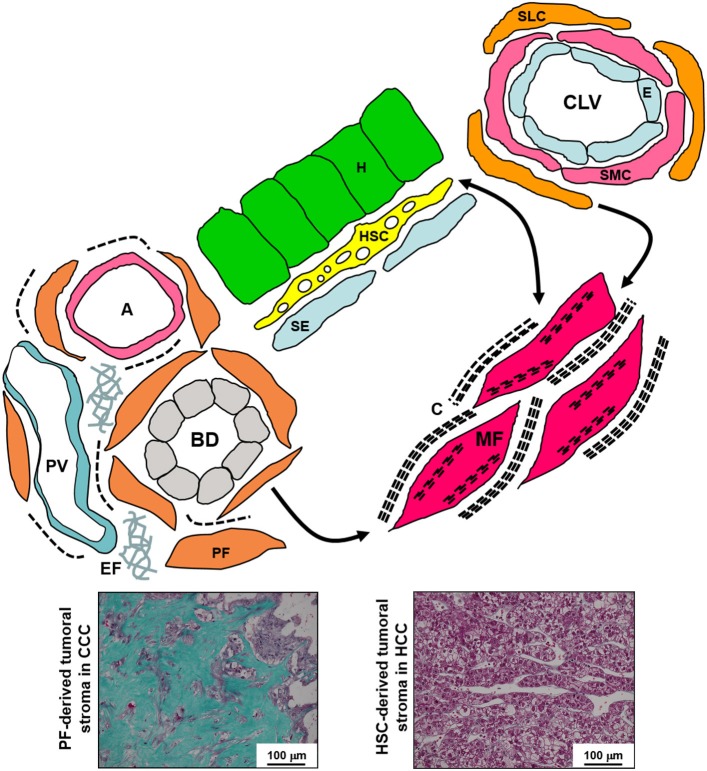
Figure 2.
Schematic diagram of the various liver fibroblastic cells able to acquire a myofibroblastic phenotype and involved in fibrogenesis and tumoral stroma formation. The portal fibroblasts (PF) located in the portal tract connective tissue around bile ducts (BD), portal arteries (A), and portal veins (PV), and the second-layer cells (SLC), fibroblasts located around the smooth muscle cells (SMC) and the endothelium (E) of the centrolobular veins (CLV), can acquire a myofibroblastic phenotype, and these cells do not seem to be able to reacquire a quiescent phenotype; in contrast, the hepatic stellate cells (HSC) containing lipids droplets and located in the Disse's space between the hepatocytes (H) and the sinusoidal endothelium (SE) can modulate their myofibroblastic differentiation, and present pericyte-like features suggesting that they function as liver-specific pericytes participating in the regulation of sinusoidal blood pressure. Myofibroblasts (MF) present microfilament bundles and secrete large amounts of extracellular matrix. In addition, PF and PF-derived MF are the major, if not the only, cells that produce elastin. Numerous PF-derived MF are involved in the formation of the abundant fibrous stroma present in cholangiocarcinoma (CCC) while generally rare HSC-derived MF are present in the scanty tumoral stroma of hepatocellular carcinoma (HCC) (Masson's trichrome histochemistry). C, collagen; EF, elastic fibers.