Fig. 2.
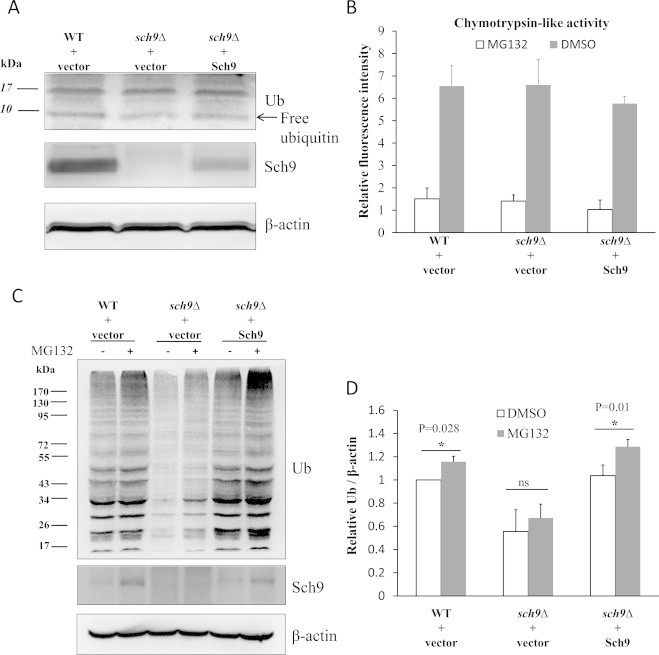
Sch9 does not regulate the level of ubiquitinated proteins by affecting ubiquitin expression or proteasomal activity. (A) Lysates of log phase yeasts (OD600 nm=0.5) were resolved on 15% SDS gels and proteins of interest were detected by Western blotting. (B) Chymotrypsin-like activity of the proteasome was monitored by Suc-Leu-Leu-Val-Tyr-AMC digestion using lysates with equal amounts of total protein from log phase yeasts at OD600nm of 0.5. The proteasome inhibitor MG132 (75 µM) was added to the isopyknic lysates as the blank control. (C) The log phase yeasts (OD600 nm=0.5) were treated with 75 µM MG132 for 1 h and the levels of ubiquitinated protein and Sch9 were tested by western blotting with actin as the loading control. (D) The quantifications of three repeats from panel C. (*represents P<0.05 and “ns” denotes no significance p>0.05 between the indicated comparisons.).
