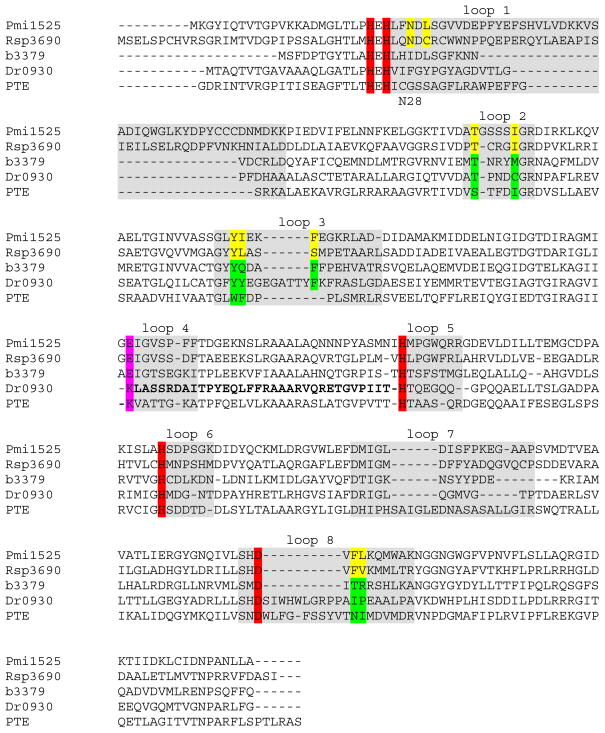
Figure 6.
Amino acid sequence alignment of Pmi1525 and four other proteins from cog1735, including the non-specific carboxylesterase Rsp3690 from subgroup 4, phosphotriesterase homology protein (PHP, b3379) from subgroup 1, γ,δ-lactonase Dr0930 from subgroup 7, and phosphotriesterase PTE from subgroup 9. The four histidine and the aspartate residues coordinated to the two metal ions are highlighted in red. The residue bridging the two metal ions is colored in pink. The residues forming the hydrophobic pocket in the active site in Pmi1525 and Rsp3690 are colored yellow and the corresponding residues in the other four proteins are colored green.