Fig. 1.
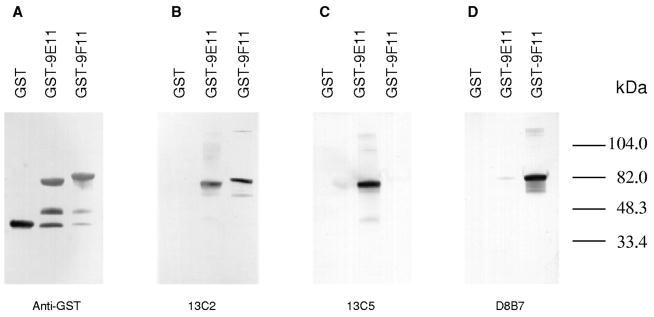
Specificity of monoclonal antibodies to recombinant spectrin Src homology 3 domain. Equal amounts of IPTG-induced bacterial lysates of the GST-SH3 domain fusion polypeptides of αI spectrin (GST-9E11, containing spectrin repeat units 9 through 11, as defined in Ref. [11]) and αII spectrin (GST-9F11, containing αII spectrin repeat units 9 through 11; it includes two sequence insertions not present in αI spectrin and accounting for an apparent difference in migration between GST-9E11 and GST-9F11) were solubilized in Laemmli buffer and separated on 7% SDS–Tricine polyacrylamide gels. Identical blotted transfers of gels were incubated with the following antibodies: anti-GST, panel A; mAb 13C2, panel B; mAb 13C5, panel C, and mAb D8B7, panel D. mAb 17C7 showed reactivity identical to mAb 13C5; mAb 15A5 was similar to mAb 13C2 but was less immunoreactive to GST-9F11. All monoclonal antibodies were raised to recombinant GST fusion polypeptides as indicated in Table 1.
