Figure 2.
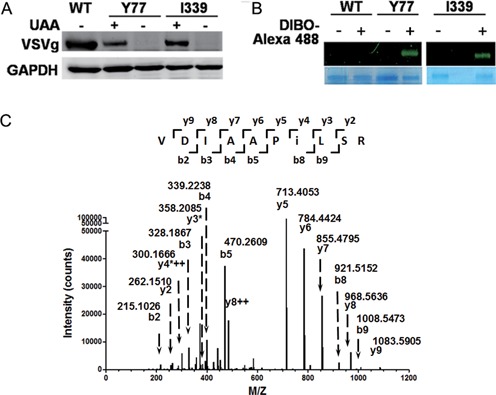
Site-specific incorporation of NAEK VSVg envelope protein. Y77 and I339 were chosen as representatives to demonstrate the compatibility of genetic code expansion with the expression of viral protein. (A) The unnatural amino acid (UAA)-dependent expression of VSVg proteins carrying NAEK at sites Y77 and I339, respectively. The absence (–) and presence (+) of UAA had different effects on VSVg expression. The expression level of VSVg was detected by western blotting analysis and GAPDH acted as an internal loading control. (B) Verification of NAEK incorporation into VSVg protein via Alexa 488 probe. Incorporation of NAEK into VSVg protein makes it possible to conjugate with DIBO-Alexa 488 via Click reaction and is thus visible. No fluorescent signal was detected for wild-type VSVg (WT) due to lack of NAEK residue. The VSVg protein, purified by IP, was incubated with (+) or without (–) DIBO-Alexa 488 at 4°C for 1 h and was then characterized by 9% SDS-PAGE. The upper bands show the corresponding fluorescent scanning results; the lower bands show the loading of VSVg protein as detected by Coomassie blue staining. (C) Verification of NAEK incorporation at the defined site (I339 as a representative) by LC-MS/MS peptide sequencing. The MS/MS fragmentation spectrum of a tryptic peptide derived from the purified protein confirm the incorporation of NAEK at I339. The mass difference of 2y4*++-y3*[2×300.1666–2-(358.2085–1) = 241.1247] corresponds well to the mass of NAEK residue (259.13–18.01 = 241.12).
