Figure 2.
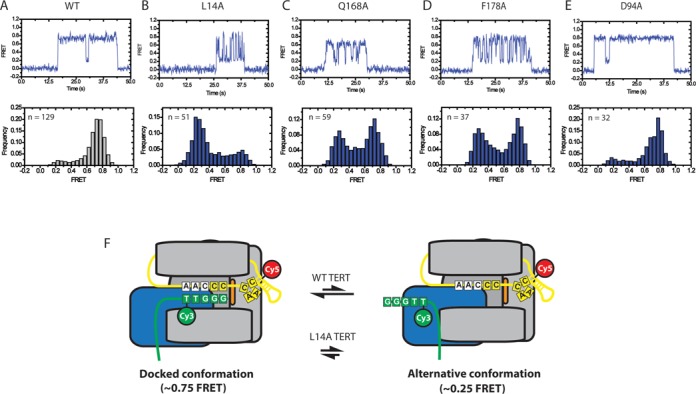
Representative smFRET traces and histograms for wild-type and mutant telomerase. (A) Representative smFRET trace (top) and smFRET histogram (bottom) for Cy3-labeled (TG)8T2G3 primer incubated with wild-type telomerase labeled with Cy5 at the U36 position of TER. Wild-type enzyme demonstrates a stable ∼0.75 FRET state with transient excursions to a ∼0.25 FRET state (top panel). This is also reflected in a smFRET histrogram of FRET values compiled from 129 separate binding events (bottom panel) demonstrating a predominant ∼0.75 FRET distribution with a small shoulder at ∼0.25 FRET. (B–E) Representative smFRET traces (top) and smFRET histograms (bottom) for L14A, Q168A and F178A mutant telomerase respectively. (F) Model of telomerase DNA binding dynamics. smFRET data indicate that DNA associated with telomerase can exist in one of at least two conformations. In the docked state, represented by the ∼0.75 FRET population, the RNA–DNA duplex is positioned in the enzyme active site. The ∼0.25 FRET population represents an alternative state that exists in an equilibrium with the docked state. In this conformation the 3′ end of the DNA is positioned away from the enzyme active site. TEN domain residues L14, Q168 and F178 bias the internal equilibrium towards the docked conformation. Importantly, smFRET alone does not provide sufficient information to fully map the contacts present in the alternative state. Therefore, although we can confidently assert that an alternative state exists, the schematic layout presented in this figure represents only one of many possible organizations that could comprise the alternative state of the enzyme.
