Figure 1.
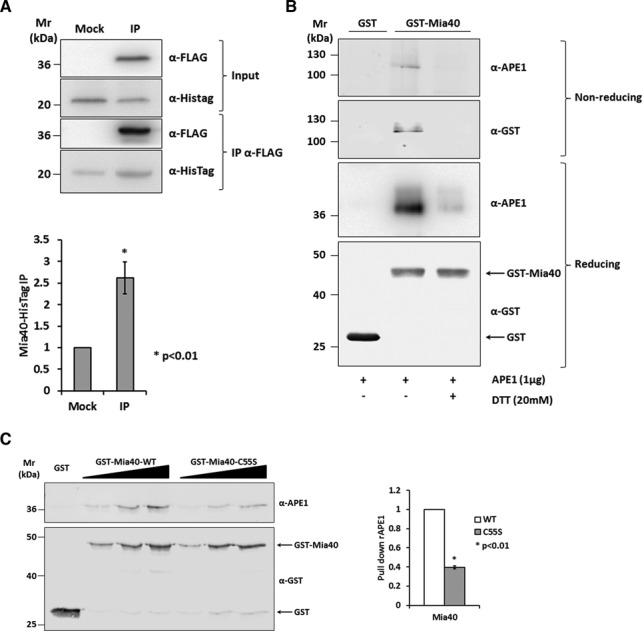
Mia40 Cys55 is responsible for the binding to APE1. (A) Western blot (top) and relative quantification (bottom) of the affinity purification analysis of HeLa cells expressing APE1-FLAG and Mia40-HisTag proteins. Control sample (Mock) and APE1-FLAG (IP) were immunoprecipitated under native conditions from total cell extracts, separated by 12% SDS-PAGE, and analyzed by western blot to evaluate the levels of each interacting partner by using specific antibodies (anti-APE1, anti-HisTag). Mia40-HisTag protein resulted enriched in IP fraction. (B) GST pull-down assay under reducing and non-reducing conditions. Fifteen picomoles of recombinant GST-Mia40 protein were used as bait, GST alone as control and APE1 protein as prey. Interaction between the two proteins was disrupted after treatment with reducing agent (DTT 20 mM). Under non-reducing conditions the complex formed by APE1 and Mia40 is visible and is disrupted when proteins were incubated with DTT. (C) Western blot (left) and relative histogram (right) of GST pull-down analysis of recombinant APE1 WT protein. GST-Mia40 WT and C55S mutant (5, 10 and 15 pmoles) were used as prey and GST (15 pmoles) alone as control. The binding of APE1 (5, 10 and 15 pmoles) was normalized to the western blotting signal of GST-Mia40 and resulted strongly decrease by the mutation of Mia40 Cys55 residue.
