Figure 2. Chromatographic evidence that plant and bacterial COG3236 proteins cleave the pyrimidine moiety from riboflavin biosynthesis intermediates 1 and 2.
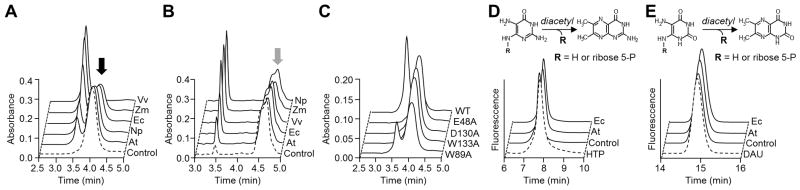
(A) Action of E. coli (Ec), N. punctiforme (Np), V. vulnificus (Vv), Arabidopsis (At), and maize (Zm) COG3236 proteins on intermediate 1 (black arrow). Reactions (50 μl) containing 3 mM intermediate 1 and 30 μg of protein were incubated for 1 h at 22°C and analyzed by HPLC with UV detection. Control reactions contained no enzyme.
(B) Action of COG3236 proteins on intermediate 2 (grey arrow). Conditions were as in A except that substrate concentration was 2.4 mM and the amounts of protein (μg) were: Vv, 5; Ec and Np, 1; Zm 0.25; At, 0.1. The shoulder on the intermediate 2 peak is due to partial resolution of the two anomers [9].
(C) Elimination or reduction of activity of E. coli COG3236 by site-directed mutagenesis of four conserved residues. A reaction with wild type (WT) protein is shown for comparison. Conditions were as in A except that 100 μg of protein was used.
(D,E) Fluorometric HPLC analysis of the diacetyl derivatives of, respectively, intermediates 1 and 2, of the products formed therefrom by E. coli or Arabidopsis COG3236, and of authentic 4-hydroxy-2,5,6-triaminopyrimidine (HTP) or 5,6-diaminouracil (DAU). Control reactions contained no enzyme.
