Table 1.
Rh-catalyzed C–H amination of 3-phenylpropylsulfamate 7.
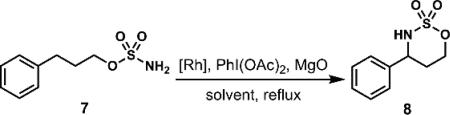
| |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Cat. load (mol%) | Solvent | Concentration (M) | Yield (%) |
| 1 | Rh2(oct)4 | 2 | DCM | 0.16 | 84 |
| 2 | Rh2(OAc)4 | 0.5 | DCM | 0.10 | n.d. |
| 3 | Rh2(OAc)4 | 0.7 | DCM | 0.20 | 72 |
| 4 | Rh2(OAc)4 | 2 | DCM | 0.15 | 81a |
| 5 | Rh2(OAc)4 | 2 | DCM | 0.15 | 93 |
| 6 | Rh2(OAc)4 | 2 | DCM | 0.15 | 85b |
| 7 | Rh2(esp)2 | 0.25 | DCE | 0.10 | n.d. |
| 8 | Rh2(esp)2 | 0.5 | DCE | 0.10 | 66a,b |
| 9 | Rh2(esp)2 | 1 | DCE | 0.10 | 72a,b |
| 10 | Rh2(esp)2 | 1 | DCE | 0.25 | 77a,b |
| 11 | Rh2(esp)2 | 1 | DCE | 0.50 | 69a,b |
| 12 | Rh2(esp)2 | 1 | DCE | 0.20 | 81a,b |
DCM = dichloromethane; DCE = 1,2-dichloroethane.
Run open to air
Purified by recrystallization
