Abstract
Background
Sensory phenomena (SP) are uncomfortable feelings, including bodily sensations, sense of inner tension, “just-right” perceptions, feelings of incompleteness, or “urge-only” phenomena, which have been described to precede, trigger or accompany repetitive behaviours in individuals with obsessive–compulsive disorder (OCD). Sensory phenomena are also observed in individuals with tic disorders, and previous research suggests that sensorimotor cortex abnormalities underpin the presence of SP in such patients. However, to our knowledge, no studies have assessed the neural correlates of SP in patients with OCD.
Methods
We assessed the presence of SP using the University of São Paulo Sensory Phenomena Scale in patients with OCD and healthy controls from specialized units in São Paulo, Brazil, and Barcelona, Spain. All participants underwent a structural magnetic resonance examination, and brain images were examined using DARTEL voxel-based morphometry. We evaluated grey matter volume differences between patients with and without SP and healthy controls within the sensorimotor and premotor cortices.
Results
We included 106 patients with OCD and 87 controls in our study. Patients with SP (67% of the sample) showed grey matter volume increases in the left sensorimotor cortex in comparison to patients without SP and bilateral sensorimotor cortex grey matter volume increases in comparison to controls. No differences were observed between patients without SP and controls.
Limitations
Most patients were medicated. Participant recruitment and image acquisition were performed in 2 different centres.
Conclusion
We have identified a structural correlate of SP in patients with OCD involving grey matter volume increases within the sensorimotor cortex; this finding is in agreement with those of tic disorder studies showing that abnormal activity and volume increases within this region are associated with the urges preceding tic onset.
Introduction
Intrusive thoughts (obsessions) and ritualized behaviours (compulsions) are the prototypical symptoms of obsessive–compulsive disorder (OCD). The classical depiction of the disorder describes obsessions as an anxiety-inducing symptom and compulsions as the behavioural response aimed at regulating or relieving the heightened anxiety levels. Nevertheless, compulsions are not always preceded by obsessions. Instead, they can be preceded, triggered or simply accompanied by uncomfortable feelings, such as physical sensations, “just-right” perceptions, feelings of incompleteness, or “urge-only” phenomena, globally referred to as sensory phenomena (SP).1–4 Importantly, the presence of SP is frequently reported, with prevalence estimates ranging from 65% to 72% of patients with OCD.5–8
In previous studies, the presence and severity of SP in patients with OCD have been related to specific clinical features, such as the presence of symmetry/ordering symptoms,6,7 early onset of the disorder,9 low insight7 and comorbidity with tics and Tourette syndrome.3,4,8,10 It is within tic disorders where, hitherto, the neural correlates of SP have been most often investigated. Thus, different studies have described the role of somatosensory and motor cortices in the process of tic generation. Wang and colleagues,11 for instance, compared a group of patients with Tourette syndrome with a control group instructed to mimic tic behaviours at a self-paced rate and described a stronger activation in the somatosensory cortex preceding spontaneous tics that modulated primary motor cortex activity. Other studies of patients with Tourette syndrome have also described greater activity in premotor and supplementary motor areas (SMA) before tic onset12,13 or a broader profile of cross-correlation between SMA and motor cortex activity preceding tic execution, suggesting an abnormally elevated level of activity in the SMA.14 Likewise, using structural MRI, Draganski and colleagues15 found a positive correlation between regional volumes within the somatosensory cortex and the intensity of premonitory sensations in patients with Tourette syndrome.
It seems, therefore, that somatosensory and motor–premotor regions are associated with the urges preceding tic onset in patients with Tourette syndrome. Nevertheless, to our knowledge, no previous studies have assessed the association between SP and sensorimotor cortex abnormalities in patients with OCD. In the present study we investigated the presence of SP in patients with OCD and quantified their severity by means of the University of São Paulo Sensory Phenomena Scale (USP-SPS).6 We aimed to compare structural brain abnormalities between patients with OCD with and without SP and the association between SP scores and the voxel-wise grey matter measurements from the sensorimotor cortex. Regional grey matter measurements provide a relatively stable marker of brain abnormality, thus allowing the identification of structural correlates of OCD16–19 or specific clinical features.20–23
We analyzed structural MRI data from 2 independent research centres located in São Paulo, Brazil, and Barcelona, Spain. Specifically, we compared regional grey matter volumes between patients with and without SP as well as between each OCD group and a control group comparable in age and sex. Likewise, to fully characterize the association between SP and brain anatomy, we also expored correlations between the severity of SP and regional grey matter volumes. We centred our analyses on a broad cortical area encompassing sensorimotor and premotor cortices of both hemispheres. On the grounds of previous findings in tic disorder samples, we hypothesized that patients with OCD with SP would present regional grey matter volume increases in comparison to patients without SP and healthy controls. Similarly, we also predicted that regional grey matter volume within this area would correlate with the intensity of SP, as assessed with the USP-SPS.
Methods
Participants
We recruited patients with OCD and healthy controls from 2 specialized OCD research units: the OCD Spectrum Disorders Program at the University of São Paulo Medical School, São Paulo, Brazil, and the OCD Clinic and Research Unit at Bellvitge University Hospital, Barcelona, Spain. All patients were required to have a primary diagnosis of OCD according to DSM-IV criteria, and they had to be treatment-naive or stably medicated for at least 3 months before MRI. Exclusion criteria were age younger than 18 or older than 65 years, current psychoactive substance abuse or current/past substance dependence, mental retardation, neurologic comorbidity, current or history of psychotic disorders, current or history of any other severe medical conditions and any contraindication to MRI scanning. All patients underwent the Structured Clinical Interview for DSM-IV Axis I Disorders — Clinician Version (SCID-CV).24 Comorbidity with other Axis I disorders, including depression and anxiety disorders, was not considered an exclusion criterion provided that OCD was the primary diagnosis and the reason for seeking medical assistance.
Healthy controls were recruited from the same sociodemographic environments (i.e., the area of influence of the hospitals) and were comparable to the patient sample in age, sex and education level. Controls were required to have no current or history of neurologic or psychiatric disorders based on the Structured Clinical Interview for DSM-IV non-patient version (SCID-NP), although quantitative assessments of depression and anxiety symptoms were not administered to control participants. The rest of exclusion criteria were the same used for the OCD group.
All participants provided written informed consent after receiving a complete description of the study and the assurance that the decision to participate in the study would not interfere with their access to treatment. The University of Sao Paulo Medical School ethical committee and the Bellvitge University Hospital ethical committee independently approved the study, which was performed in accordance with the Declaration of Helsinki.
Clinical assessment
Each patient was administered the Yale–Brown Obsessive–compulsive Scale (Y-BOCS),25 the Dimensional Yale–Brown Obsessive–compulsive Scale (DY-BOCS)26 and the USP-SPS.6 The USP-SPS is a semistructured instrument that was developed to examine the presence and severity of different types of SP that precede or accompany repetitive behaviours. The USP-SPS is divided in 2 parts: a checklist and a severity scale. The checklist is composed of items evaluating past and current examples of different subtypes of SP, including
physical sensations characterized by uncomfortable feelings localized in specific body regions (skin, muscles, joints);
“just right” perceptions triggered by tactile, visual, or auditory sensations and involving a strong need for things to feel perfect or “just right” (e.g., a need to touch objects or people, a need for objects to look a certain way [e.g., perfectly symmetric], and/or a need for a person’s voice or an audio recording to sound perfect or have the perfect pitch);
feelings of incompleteness/need to feel internally “just right” (an inner feeling and/or perception of discomfort that leads the patient to complete certain movements or actions until the feeling is relieved);
energy sensations, or a generalized inner tension that builds up and needs to be released by completing a movement or an action; and
“urges-only” phenomena, or a strong urge to engage in a repetitive behaviour without any specific preceding feeling or sensation.
The severity scale evaluates the severity of the SP using 3 ordinal scales, each with scores from 0 to 5, that focus on the frequency of the SP, the amount of distress they cause and the degree to which they interfere with the patient’s functioning. The total severity score is obtained by summing these 3 scores, for a total posssible score of 0–15.
MRI acquisition
Brain images from both centres were acquired with a 1.5 T Signa scanner (General Electric) according to the following protocols. In São Paulo, contiguous 1.6 mm axial high-resolution T1-weighted anatomic images were obtained from each participant across the entire brain using a T1–3-dimensional spoiled gradient recalled acquisition (T1–3D SPGR) sequence, with the following acquisition parameters: echo time (TE) 4.2 ms, repetition time (TR) 10.5 ms, flip angle 15°, acquisition matrix 256 × 192. Images were then interpolated using ZIP2 to a final voxel size of 0.94 × 0.94 × 0.80 mm (248 slices). In Barcelona, contiguous 1.2 mm axial high-resolution T1-weighted anatomic images were obtained from each participant across the entire brain using a T1–3D SPGR sequence, with the following acquisition parameters: TE 4.2 ms, TR 11.8 ms, flip angle 90°, acquisition matrix 256 × 256 and a final voxel size of 1.17 × 1.17 × 1.2 mm (130 slices).
Image preprocessing and statistical analysis
After inspection for the presence of artifacts, the MRI data were preprocessed using the optimized voxel-based morphometry (VBM)-DARTEL27 pipeline implemented in the SPM8 software (Wellcome Trust Centre for Neuroimaging). Specifically, the preprocessing protocol involved tissue segmentation, normalization and smoothing. Image segmentation was performed using the “new segment” algorithm, obtaining grey matter image segments from native-space MRIs. Nevertheless, we discarded final output images from this preprocessing step and reserved the rigidly transformed versions to be used for DARTEL normalization. Thus, with the “create templates” function, images were iteratively matched to a template generated from their own mean in order to generate a series of templates with increasing resolution. Subsequently, native space grey matter images were registered to the highest resolution grey matter template within a high-dimensional diffeomorphic framework and resampled to a final voxel size of 1.5 mm3. Spatially normalized tissue maps were then modulated using the Jacobian determinants derived from the corresponding flow fields to restore volumetric information. Finally, images were smoothed with a 10 mm full-width at half-maximum isotropic Gaussian kernel to account for the high across-subject anatomic variability of cortical structures, such as our region of interest.
Since this was a hypothesis-driven study, analyses were spatially restricted with an image mask including sensorimotor and premotor areas (Brodmann areas [BA] 1–6) by using the Wake Forest University (WFU) Pickatlas.28 We carried out between-group comparisons of modulated grey matter coefficients using a general linear model in which group (control, OCD with SP and OCD without SP) was entered as the variable of interest, with site, age, sex and total grey matter volume as nuisance covariates. Likewise, to assess correlations between regional grey matter volumes and SP severity, we performed a multiple regression analysis with the USP-SPS score as the regressor of interest and site, age, sex and total grey matter volume as nuisance covariates (this analysis was restricted to the OCD with SP group). In both analyses, statistical significance was established by combining voxel- and cluster-level thresholds. The cluster extent threshold was determined using the AlphaSim function implemented in the SPM-REST toolbox29 by means of 1000 Monte Carlo simulations, with a voxel-level significance of p < 0.01 and a cluster connection radius of 3 mm within a grey matter mask of 55 785 voxels and the actual smoothing of the data after model estimation. This resulted in a minimum spatial cluster extent ranging from 521 to 572 voxels (depending on the specific analysis) to satisfy a family-wise error (FWE) rate of p < 0.05. However, the resulting cluster extent was further adjusted to account for the nonisotropic smoothness of VBM images in accordance with the study by Hayasaka and colleagues.30
We performed a series of post hoc analyses to rule out the potential effects of different confounding variables. Specifically, because lifetime history of comorbid depression or anxiety disorders and medication status have been shown to affect structural measurements in patients with OCD (for example, see the reviews by Piras and colleagues31,32), we controlled for these potential confounding effects. Likewise, we also controlled for the potential effect on our imaging results of tic disorders and the variables associated with the presence or severity of SP.
We conducted exploratory whole brain analyses to detect possible structural correlates of SP outside sensorimotor–premotor cortices. These analyses were like the ones reported above, but not restricted to our region of interest. Hence, statistical significance was also established by combining voxel- and cluster-level thresholds, although we used a whole brain mask instead of the sensorimotor–premotor mask. This resulted in a minimum spatial cluster extent ranging from 1420 to 1534 voxels (depending on the specific analysis) to satisfy an FWE rate of p < 0.05. Such a cluster extent was also further adjusted to account for the nonisotropic smoothness of VBM images.
Finally, we carried out clinical and demographic comparisons using SPSS software version 19. Differences in clinical variables were assessed using χ2 and independent samples t tests for categorical and continuous variables, respectively. We also calculated Pearson correlations to further characterize the association between continuous variables and the severity of SP. In these analyses, we considered results to be significant at p < 0.05, 2-tailed.
Results
Clinical and demographic characteristics
Altogether, 106 patients with OCD (51 from Brazil, 55 from Spain) and 87 healthy controls (37 from Brazil, 50 from Spain) were assessed in the present study. Table 1 summarizes the sociodemographic data from broth groups and the clinical data from the OCD sample. Of the 106 participants with OCD, 71 (67%) reported at least 1 type of SP preceding and/or accompanying the compulsions. Patients with and without SP did not differ in terms of age, sex, handedness, years of education, age at onset of OCD symptoms, illness duration, global severity scores (Y-BOCS and DY-BOCS), or history of tics. The severity of the different OCD symptom dimensions did not significantly differ between the OCD groups, with the exception of the symmetry/ordering dimension, which was more severe in patients with SP (p < 0.001). The most frequent subtype of SP was the presence of “just right” perceptions (85%), followed by physical sensations (56%), feeling of incompleteness (44%), urges (30%) and energy release (24%). These results are summarized in Table 2.
Table 1.
Clinical and demographic characteristics of patients with obsessive–compulsive disorder and healthy controls
| Group, mean ± SD or no. (%) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | OCD, n = 106 | Control, n = 87 | Statistic | p value |
| Age, yr | 33.11 ± 9.37 | 32.13 ± 9.57 | t191 = 0.72 | 0.47 |
| Age at onset of OCS, yr* | 17.0 ± 8.85 | — | — | — |
| Illness duration, yr* | 16.08 ± 10.29 | — | — | — |
| Y-BOCS | ||||
| Obsessions | 13.0 ± 3.20 | — | — | — |
| Compulsions | 13.45 ± 2.97 | — | — | — |
| Total score | 26.46 ± 5.86 | — | — | — |
| Global DY-BOCS score† | 22.37 ± 4.28 | — | — | — |
| DY-BOCS severity scores† | ||||
| Aggression‡ | 6.58 ± 5.20 | — | — | — |
| Sexual/religious§ | 3.67 ± 5.27 | — | — | — |
| Symmetry¶ | 7.24 ± 5.44 | — | — | — |
| Contamination | 6.34 ± 5.74 | — | — | — |
| Hoarding | 2.93 ± 4.10 | — | — | — |
| Miscellaneous** | 7.18 ± 5.59 | — | — | — |
| Female sex | 56 (52.8) | 49 (56.3) | χ21 = 0.24 | 0.67 |
| Years of education | χ22 = 4.23 | 0.12 | ||
| 9–13 | 62 (58.5) | 39 (44.8) | ||
| 14–18 | 37 (34.9) | 36 (41.4) | ||
| 19–24 | 7 (6.6) | 12 (13.8) | ||
| Right-handed | 98 (92.4) | 86 (98.8) | χ22 = 3.89 | 0.14 |
| Lifetime history of tics | 18 (17.0) | — | — | — |
| Lifetime history of depression | 41 (38.7) | — | — | — |
| Lifetime history of anxiety disorders | 39 (36.8) | — | — | — |
DY-BOCS = Dimensional Yale–Brown Obsessive–compulsive Scale; OCD = obsessive–compulsive disorder; OCS = obsessive–compulsive symptoms; SD = standard deviation; Y-BOCS = Yale–Brown Obsessive–Compulsive Scale.
One missing value.
Two missing values.
Obsessions about harm due to aggression, injury, violence, natural disasters and related compulsions.
Sexual, moral and religious obsessions and related compulsions.
Includes compulsions to count or order/arrange.
Includes obsessions and compulsions about somatic concerns and superstitions, among other symptoms not classified in the previous symptom dimensions.
Table 2.
Clinical and demographic characteristics of patients with obsessive–compulsive disorder with and without sensory phenomena
| Group, mean ± SD or no. (%) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | OCD with SP, n = 71 | OCD without SP, n = 35 | Statistic | p value |
| Age, yr | 32.11 ± 8.45 | 35.14 ± 10.84 | t104 = −1.56 | 0.12 |
| Age at onset of OCS, yr* | 16.79 ± 9.85 | 17.43 ± 6.49 | t103 = −0.35 | 0.73 |
| Illness duration, yr* | 15.26 ± 8.89 | 17.71 ± 12.61 | t103 = −1,16 | 0.25 |
| Y-BOCS | ||||
| Obsessions | 12.99 ± 3.38 | 13.03 ± 2.85 | t104 = −0.06 | 0.95 |
| Compulsions | 13.62 ± 3.02 | 13.11 ± 2.89 | t104 = 0.82 | 0.41 |
| Total score | 26.61 ± 5.99 | 26.17 ± 5.64 | t104 = 0.36 | 0.72 |
| Global DY-BOCS score† | 22.44 ± 4.49 | 22.21 ± 3.85 | t102 = 0.25 | 0.81 |
| DY-BOCS severity scores†‡ | ||||
| Aggression | 6.31 ± 5.40 | 7.15 ± 4.76 | t102 = −0.77 | 0.45 |
| Sexual/religious | 3.18 ± 5.21 | 4.73 ± 5.31 | t102 = −1.40 | 0.17 |
| Symmetry | 8.65 ± 5.06 | 4.21 ± 5.03 | t102 = 4.17 | < 0.001 |
| Contamination | 6.17 ± 5.88 | 6.70 ± 5.52 | t102 = −0.44 | 0.67 |
| Hoarding | 3.34 ± 4.23 | 2.06 ± 3.72 | t102 = 1.49 | 0.14 |
| Miscellaneous | 7.11 ± 5.78 | 7.12 ± 5.31 | t102 = −0.01 | 0.85 |
| Total severity of SP | 8.35 ± 3.46 | |||
| Female sex | 36 (50.7) | 20 (57.1) | χ21 = 0.39 | 0.53 |
| Years of education | χ22 = 1.09 | 0.58 | ||
| 9–13 | 42 (59.1) | 21 (60.0) | ||
| 14–18 | 23 (32.4) | 13 (37.1) | ||
| 19–24 | 6 (8.5) | 1 (2.9) | ||
| Right-handed | 65 (91.5) | 34 (97.1) | χ22 = 5.14 | 0.08 |
| Lifetime history of tics | 10 (14.1) | 8 (22.9) | χ21 = 1.28 | 0.28 |
| Lifetime history of depression | 31 (43.7) | 10 (28.6) | χ21 = 2.25 | 0.15 |
| Lifetime history of anxiety disorders | 28 (39.4) | 11 (31.4) | χ21 = 0.65 | 0.52 |
| Treatment-naive | 29 (40.8) | 11 (31.4) | χ21 = 0.89 | 0.39 |
| Subtypes of SP (lifetime) | ||||
| Physical sensations | 40 (56.3) | — | — | — |
| “Just right” perceptions | 60 (84.5) | — | — | — |
| Feeling of incompleteness | 31 (43.7) | — | — | — |
| Energy release | 17 (23.9) | — | — | — |
| “Urge-only” phenomena | 21 (29.6) | — | — | — |
DY-BOCS = Dimensional Yale–Brown Obsessive–Compulsive Scale; OCD = obsessive–compulsive disorder; OCS = obsessive–compulsive symptoms; SD = standard deviation; SP = sensory phenomena; USP-SPS = University of São Paulo Sensory Phenomena Scale; Y-BOCS, Yale–Brown Obsessive–Compulsive Scale.
One missing value.
Two missing values.
See Table 1 footnote for a description of each symptom dimensions profile.
Among patients with SP, the mean total severity of SP was 8.4 ± 3.5. Moreover, we observed a positive correlation between SP severity and symmetry/ordering severity (r = 0.24, p = 0.040), DY-BOCS (r = 0.39, p = 0.001) and Y-BOCS scores (obsessions subscale: r = 0.29, p = 0.013; compulsions subscale: r = 0.24, p = 0.045; total score: r = 0.28, p = 0.016). No other significant correlations were observed.
Brazilian patients with OCD were younger than the Spanish patients (30.43 ± 8.46 yr v. 35.60 ± 9.55 yr, t = 2.94, p = 0.004). Importantly, however, Brazilian and Spanish subsamples did not differ in frequency of SP, although severity of SP was greater in the Spanish than in the Brazilian subsample (9.17 ± 2.72 v. 7.56 ± 3.93, t = 2.02, p = 0.048). Conversely, global severity measurements (DY-BOCS and Y-BOCS scores) did not differ between sites.
Imaging analyses
In the direct comparison between the 2 groups of patients with OCD, those with SP showed a significant grey matter volume increase in 2 clusters located in the left and right medial sensorimotor cortices (Fig. 1 and Table 3). Conversely, in the analyses assessing putative correlations between regional grey matter volumes and severity of SP, we did not observe any significant association within our region of interest.
Fig. 1.
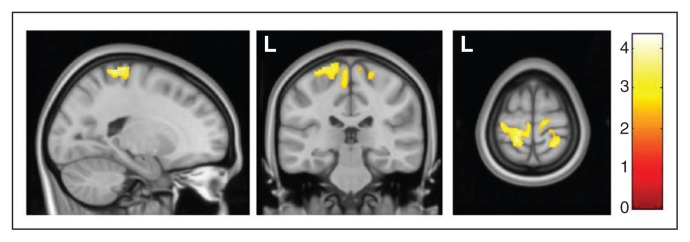
Increased regional grey matter volumes in patients with obsessive–compulsive disorder with sensory phenomena (SP) in comparison to patients without SP in the bilateral sensorimotor cortex. Colour bar represents t values. L = left hemisphere.
Table 3.
Regions with significant grey matter volume alterations characterizing patients with obsessive–compulsive disorder with sensory phenomena
| Contrast | Peak MNI coordinate x, y, z | t (Z) | k | p value (uncorrected) | p value (corrected)* | Localization |
|---|---|---|---|---|---|---|
| SP v. no SP | −15, −31, 70 | 3.92 (3.84) | 1420 | < 0.001 | < 0.001 | Left sensorimotor cortex |
| 26, −43, 72 | 4.34 (4.23) | 769 | < 0.001 | 0.012 | Right sensorimotor cortex | |
| SP v. control | −24, −28, 70 | 4.63 (4.50) | 1541 | < 0.001 | < 0.001 | Left sensorimotor cortex |
| 12, −33, 73 | 3.71 (3.64) | 543 | < 0.001 | 0.039 | Right sensorimotor cortex |
MNI = Montreal Neurological Institute; SP = sensory phenomena.
Multiple comparisons correction was performed by combining voxel- and cluster-level significance thresholds using AlphaSim (see article for details).
In different post hoc analyses we evaluated the confounding effects of tic disorders, lifetime history of comorbid depression or anxiety disorders and medication status. Thus, when repeating the analyses after excluding patients who reported a lifetime history of tics (n = 18), depression (n = 41) or other anxiety disorders (n = 39), between-group differences in sensorimotor grey matter content remained significant. Similarly, we also controlled for the effect of medication by excluding those patients taking medication (n = 66), and our results remained significant. Also, we assessed for the potential confounding effects of the variables significantly associated with presence or severity of SP. In this case, analyses were repeated by controlling for the presence of symmetry/ordering symptoms, total severity of symmetry/ordering symptoms and overall disorder severity measurements (global DY-BOCS and the different scores of the Y-BOCS). In all cases, the differences between OCD groups remained unchanged. Furthermore, none of these confounders was correlated with grey matter volume within the sensorimotor cortex region (all p > 0.05).
We further explored potential volume differences between the OCD groups by assessing their respective volume changes compared with healthy controls. Compared with the control group, patients with SP showed increased grey matter volume in 2 clusters bilaterally covering the medial aspect of the sensorimotor cortex (Fig. 2 and Table 3). By contrast, no significant differences were observed between patients with OCD without SP and the control group.
Fig. 2.
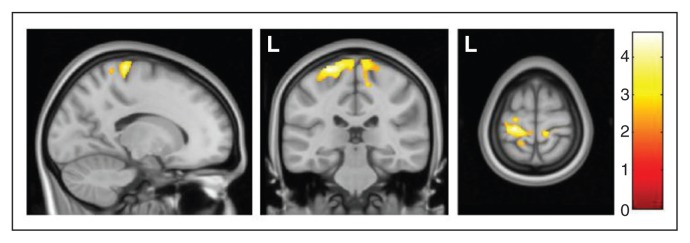
Increased regional grey matter volumes in patients with obsessive–compulsive disorder with sensory phenomena in comparison to healthy controls in the bilateral sensorimotor cortex. Colour bar represents t values. L = left hemisphere.
Finally, exploratory whole brain analyses did not reveal any significant difference outside our region of interest between patients with and without SP. By contrast, we observed a significant volume increase in patients with SP compared with healthy controls in a large cluster encompassing the left posterior putamen, pallidum, thalamus (mainly the ventral posterolateral nucleus) and the mesencephalic locomotor region (Fig. 3). Such changes were not observed in patients without SP.
Fig. 3.
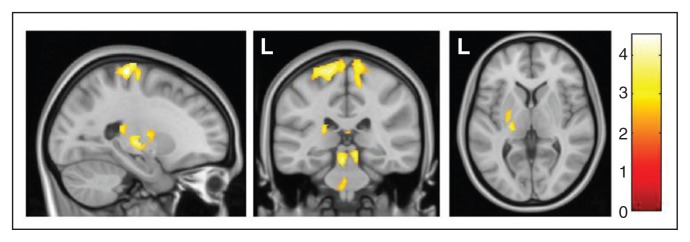
Whole brain analyses showed a regional grey matter volume increase in patients with obsessive–compulsive disorder with sensory phenomena in comparison to healthy controls in a large cluster encompassing the left posterior putamen, pallidum, thalamus and mesencephalic locomotor region (peak Montreal Neurological Institute coordinates: x, y, z = −5, −30, −18; t = 3.84, p < 0.05). The sensorimotor grey matter volume increase reported in the region of interest analysis can also be observed in the sagittal and coronal slices. Colour bar represents t values. L = left hemisphere.
Discussion
We analyzed the brain structural correlates of SP in a large sample of patients with OCD. We centred our analyses in the sensorimotor cortex, because previous studies performed in tic disorder samples suggested that activity within sensorimotor regions underpins the SP that precede tic onset. In agreement with our hypotheses, we observed a significant grey matter volume increase in the medial aspect of the sensorimotor cortex in patients with SP in comparison to those without SP. Likewise, such a grey matter volume increase was also observed between patients with SP and healthy controls. Conversely, patients without SP did not differ from healthy controls. Such comparisons with healthy controls helped us to interpret the differences between the 2 patient groups, indicating that these differences were indeed the consequence of an abnormal grey matter volume increase in patients with SP rather than of a grey matter volume reduction in patients without SP. We did not, however, observe any significant correlation between regional grey matter volumes and SP severity, which indicates that although sensorimotor volume increases are clearly associated with the presence of SP, the severity of such symptoms may be modulated by other clinical or neurobiological factors.
In our sample, 67% of patients with OCD reported the presence of some SP preceding or accompanying their compulsions. This frequency of SP in patients with OCD is in agreement with data from previous studies, where frequency estimates ranged from 65% to 72%.5–8 Moreover, in our patients with SP, “just-right” perceptions (triggered by tactile, visual, or auditory sensations) were the most frequently reported symptom, followed by physical sensations, feelings of incompleteness, “urge-only” phenomena and energy release. Although literature in the area is not extensive, such distribution of the type of SP is also in agreement with previous reports.6,7 Regarding the association of SP with other clinical variables, it is worth noting that in our sample the presence of SP was not associated with a lifetime history of tic disorders. In any case, to further confirm that our imaging findings were exclusively related to the presence of SP, we repeated the analyses after excluding patients with a positive history of tic disorders, and our results remained significant. Indeed, in Tourette syndrome samples, the neural systems related to tic severity, as assessed by the Yale Global Tic Severity Scale (YGTSS),33 and the severity of premonitory sensations, as assessed by the Premonitory Urges for Tics Scale (PUTS),34 have been clearly dissociated. Thus, while tic severity has been associated with grey matter reductions in the orbitofrontal and ventrolateral prefrontal cortices, the severity of premonitory sensations has been related to volume increases in somatosensory regions.15 Our results concur with such findings and support the interpretation of SP in patients with OCD as independent from the presence of tics, but related to the same neural systems involved in the regulation of premonitory urges in tic disorder samples.
The presence of SP seem to be also largely unrelated to the most relevant clinical features of OCD, such as severity, comorbidities, age at onset, or illness duration. Such lack of clinical differences between patients with and without SP is in partial disagreement with previous reports;6,7,35 therefore, further research is warranted to evaluate the association between different clinical features and the presence of SP across different OCD samples. Nevertheless, when we centred our analyses on the group of patients with SP, we observed a significant correlation between the severity of SP and global disorder severity. This finding, in combination with our lack of correlation between SP severity and sensorimotor grey matter content, suggests that SP severity may relate to more general clinical and neurobiological factors, not necessarily to sensorimotor cortex function. Likewise, we have replicated the previously described association of SP with symmetry/ordering symptoms,6,7 although we confirmed that our sensorimotor grey matter increases did not depend on these symptoms. Despite a previous study reporting that symmetry/ordering symptoms were related to grey matter alterations in a sensorimotor region,22 this finding pertained to a more lateral (in relation to our findings) region of the right hemisphere. Moreover, in that study, greater severity of symmetry/ordering symptoms predicted lower grey matter volume, whereas based on our data we would have predicted the opposite. In addition, other studies have reported no associations between symmetry/ordering symptoms and brain anatomy.21,23 Consequently, although the association between the presence of SP and symmetry/ordering symptoms seems to be a robust finding, the neural correlates of these symptoms should probably be evaluated while controlling for the presence of SP and other confounding factors.
Morphometric alterations in the sensorimotor cortex have seldom been reported in OCD samples, and the few existing findings are quite heterogeneous, ranging from volume36 and thickness37 decreases to grey matter density increases.38 Our findings, however, are derived from a clear regional hypothesis and limited to a subgroup of patients, which may account for the scarcity of significant findings within the sensorimotor cortex in exploratory whole-brain analyses assessing general OCD populations. In this context, our results may be interpreted in terms of structural plasticity39 distinctively occurring in the sensorimotor cortex of patients with SP. Specifically, sensorimotor cortex volume increases may depend on neural tissue enlargements stemming from increased somatosensory processing demands and the subsequent integration of this information into repetitive and ritualized motor behaviours.15 Alternatively, sensorimotor grey matter volume increases could precede the onset of disorders, thus conferring a specific vulnerability to increased sensorimotor activity. Unfortunately, the cross-sectional nature of the present study does not allow us to reach an unambiguous conclusion on this issue. In any case, both possibilities are in agreement with electrophysiological evidence of sensorimotor hyperexcitability and altered sensory gating observed in general OCD samples.40,41 Moreover, somatosensory cortex hyperactivity and the prominent influence of somatosensory information over motor cortex activity has been consistently reported in association with the presence of SP,11–14 and neural activity in the sensorimotor area has been related to urge sensations in other contexts, such as voluntary blink suppression,42 urge to void,43 or urge to cough.44 Likewise, it should be highlighted that while in Tourette syndrome samples grey matter increases have been located in more lateral regions of the somatosensory cortex (corresponding to the face and speech related regions),15 in our study grey matter increases were observed in more medial regions, corresponding to the trunk and appendicular body regions, where most of the bodily SP are experienced by patients with OCD.
Despite our exploratory whole brain analysis not detecting any significant difference between patients with and without SP, we observed a significant volume increase in patients with SP compared with healthy controls involving different subcortical sensorimotor regions, from the posterior putamen to the ventral posterolateral nucleus of the thalamus, the somatosensory thalamic relay. Such findings allow interpretation at the network level, with alterations also involving subcortical sensorimotor processing regions, concurring with previous reports suggesting that impaired sensorimotor integration in patients with OCD stems from an abnormal subcortical drive.40 Likewise, subcortical alterations extended to the mesencephalic locomotor region, which includes the cuneiform, subcuneiform and pedunculopontine nuclei.45 Interestingly, this later nucleus is critically involved in prepulse inhibition,46 a test of sensorimotor gating that has been found to be altered in patients with OCD.47
Limitations
Our study is not without limitations. First, some of our patients with OCD were taking medication, although doses were stable in the 3 months preceding MRI acquisition. Results, however, remained significant after controlling for this confounding factor. Second, we did not evaluate the presence of SP in healthy controls, which might be considered a study limitation because the presence of SP may be understood from a dimensional perspective. Nevertheless, the main objective of the study was the comparison between patients with and without SP, whereas the comparison with healthy controls was performed to obtain a reference “normative” value that helped with the interpretation of findings. Finally, patients were recruited and scanned in 2 different OCD-specialized psychiatry units, which may raise some methodological concerns in relation to the homogeneity of the clinical assessments and the imaging acquisition protocols. However, all clinical assessments were performed using standardized procedures, and despite the existence of some differences in the MRI acquisition parameters, images were preprocessed and analyzed together using standard preprocessing and analysis algorithms. Indeed, these 2 OCD units have already shared data in other multicentric studies involving structural MRI in patients with OCD,18 and it has been shown that the advantages of these kind of studies (e.g., increased statistical power and common preprocessing and analysis algorithms) exceed the putative limitations associated with differences in data acquisition protocols.
Conclusion
We have identified a structural correlate of SP in patients with OCD consisting of grey matter volume increases in the sensorimotor cortex. Speculatively, such a volume increase may be due to structural plasticity induced by an increased demand in somatosensory information processing, also affecting motor cortex activity. Alternatively, abnormally increased grey matter volumes in the sensorimotor cortex might precede the onset of the disorder, and in that case sensorimotor alterations would predispose to the occurrence of SP. Longitudinal assessments and studies invovling patients with recently diagnosed OCD are warranted to elucidate this issue.
Sensorimotor grey matter volume increases seem to be a common mechanism underlying the presence of SP in patients with different disorders, and in those with OCD these increases seem not to be necessarily related to the presence of tics, other comorbidities, or greater OCD severity. The identification of the sensorimotor cortex as the region underpinning the experience of SP in patients with OCD may help in the development of specific therapeutic approaches aimed at regulating neural activity, such as transcranial magnetic or direct current stimulation of motor or premotor regions, which have already been shown to normalize hyperactivity within the sensorimotor cortex and ameliorate overall symptom severity in patients with OCD.48
Acknowledgements
This study received financial support from the Carlos III Health Institute and the Agència de Gestió d’Ajuts Universitaris i de Recerca-AGAUR (Spain; grant nos. PI09/01331, CP10/00604, PI10/01753, PI12/01306, PI13/00918, PI13/01958, PI14/00413, CIBER-CB06/03/0034, 2014SGR1672), and from a grant of the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Foundation for the Support of Research in the State of São Paulo, Brazil) to E. Miguel (2011/21357-9). E. Miguel has also received support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, National Council for Scientific and Technological Development) (302463/2011-9). J. Sato is supported by grants from FAPESP (2013/10498-6 and 2013/00506-1). J. Diniz is supported by a postdoctoral scholarship from FAPESP (2011/00968-0). R. Assis is supported by a scientific initiation scholarship from FAPESP (2013/22536-0). M. Hoexter is supported by a postdoctoral scholarship from FAPESP (2013/16864-4). M. Subirà and E. Cerrillo are supported by a predoctoral grant from IDIBELL (Bellvitge Biomedical Research Institute). C. Soriano-Mas is funded by a Miguel Servet contract from the Carlos III Health Institute (CP10/00604).
Footnotes
Competing interests: None declared.
Contributors: M. Subirà, M. do Rosário, J. Menchón, G. Busatto, N. Cardoner, E. Miguel, M. Hoexter and C. Soriano-Mas designed the study. M. Subirà, P. Alonso, C. Segalàs, M. Batistuzzo, E. Real, A. Lopes, E. Cerrillo, J. Diniz, J. Pujol, R. Assis, J. Menchón, G. Busatto, E. Miguel, M. Hoexter and C. Soriano-Mas acquired the data, which M. Subirà, J. Sato, P. Alonso, C. Segalàs, E. Real, J. Menchón, R. Shavitt, N. Cardoner, M. Hoexter and C. Soriano-Mas analyzed. M. Subirà, M. do Rosário, M. Hoexter and C. Soriano-Mas wrote the article, which all authors reviewed and approved for publication.
References
- 1.Miguel EC, Coffey BJ, Baer L, et al. Phenomenology of intentional repetitive behaviors in obsessive-compulsive disorder and Tourette’s disorder. J Clin Psychiatry. 1995;56:246–55. [PubMed] [Google Scholar]
- 2.Miguel EC, Baer L, Coffey BJ, et al. Phenomenological differences appearing with repetitive behaviors in obsessive-compulsive disorder and Gilles de la Tourette’s syndrome. Br J Psychiatry. 1997;170:140–5. doi: 10.1192/bjp.170.2.140. [DOI] [PubMed] [Google Scholar]
- 3.Miguel EC, Rosario-Campos MC, Prado H, et al. Sensory phenomena in obsessive-compulsive disorder and Tourette’s disorder. J Clin Psychiatry. 2000;61:150–6. doi: 10.4088/jcp.v61n0213. [DOI] [PubMed] [Google Scholar]
- 4.Prado HS, do Rosário MC, Lee J, et al. Sensory phenomena in obsessive-compulsive disorder and tic disorders: a review of the literature. CNS Spectr. 2008;13:425–32. doi: 10.1017/s1092852900016606. [DOI] [PubMed] [Google Scholar]
- 5.Lee JC, Prado HS, Diniz JB, et al. Perfectionism and sensory phenomena: phenotypic components of obsessive-compulsive disorder. Compr Psychiatry. 2009;50:431–6. doi: 10.1016/j.comppsych.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Rosario MC, Prado HS, Borcato S, et al. Validation of the University of São Paulo Sensory Phenomena Scale: initial psychometric properties. CNS Spectr. 2009;14:315–23. doi: 10.1017/s1092852900020319. [DOI] [PubMed] [Google Scholar]
- 7.Ferrão YA, Shavitt RG, Prado H, et al. Sensory phenomena associated with repetitive behaviors in obsessive-compulsive disorder: an exploratory study of 1001 patients. Psychiatry Res. 2012;197:253–8. doi: 10.1016/j.psychres.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Gomes de Alvarenga P, de Mathis MA, Dominguez Alves AC, et al. Clinical features of tic-related obsessive-compulsive disorder: results from a large multicenter study. CNS Spectr. 2012;17:87–93. doi: 10.1017/S1092852912000491. [DOI] [PubMed] [Google Scholar]
- 9.Rosario-Campos MC, Leckman JF, Mercadante MT, et al. Adults with early-onset obsessive-compulsive disorder. Am J Psychiatry. 2001;158:1899–903. doi: 10.1176/appi.ajp.158.11.1899. [DOI] [PubMed] [Google Scholar]
- 10.Leckman JF, Walker DE, Goodman WK, et al. “Just right” perceptions associated with compulsive behavior in Tourette’s syndrome”. Am J Psychiatry. 1994;151:675–80. doi: 10.1176/ajp.151.5.675. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Maia TV, Marsh R, et al. The neural circuits that generate tics in Tourette’s syndrome. Am J Psychiatry. 2011;168:1326–37. doi: 10.1176/appi.ajp.2011.09111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohlhalter S, Goldfine A, Matteson S, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–37. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- 13.Neuner I, Werner CJ, Arrubla J, et al. Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Front Hum Neurosci. 2014;8:362. doi: 10.3389/fnhum.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hampson M, Tokoglu F, King RA, et al. Brain areas co-activating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry. 2009;65:594–9. doi: 10.1016/j.biopsych.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draganski B, Martino D, Cavanna AE, et al. Multispectral brain morphometry in Tourette syndrome persisting into adulthood. Brain. 2010;133:3661–75. doi: 10.1093/brain/awq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pujol J, Soriano-Mas C, Alonso P, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:720–30. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- 17.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 18.de Wit SJ, Alonso P, Schweren L, et al. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry. 2014;171:340–9. doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- 19.Parrado-Hernández E, Gómez-Verdejo V, Martínez-Ramón M, et al. Discovering brain regions relevant to obsessive-compulsive disorder identification through bagging and transduction. Med Image Anal. 2014;18:435–48. doi: 10.1016/j.media.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Cardoner N, Soriano-Mas C, Pujol J, et al. Brain structural correlates of depressive comorbidity in obsessive-compulsive disorder. Neuroimage. 2007;38:413–21. doi: 10.1016/j.neuroimage.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert AR, Mataix-Cols D, Almeida JR, et al. Brain structure and symptom dimension relationships in obsessive-compulsive disorder: a voxel-based morphometry study. J Affect Disord. 2008;109:117–26. doi: 10.1016/j.jad.2007.12.223. [DOI] [PubMed] [Google Scholar]
- 22.Van den Heuvel OA, Remijnse PL, Mataix-Cols D, et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132:853–68. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- 23.Alvarenga PG, do Rosário MC, Batistuzzo MC, et al. Obsessive-compulsive symptom dimensions correlate to specific gray matter volumes in treatment-naïve patients. J Psychiatr Res. 2012;46:1635–42. doi: 10.1016/j.jpsychires.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 24.First N, Spitzer R, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press, Inc; 1997. [Google Scholar]
- 25.Goodman WK, Price LH, Rasmussen SA, et al. The Yale–Brown Obsessive–compulsive Scale I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 26.Rosario-Campos MC, Miguel EC, Quatrano S, et al. The Dimensional Yale-Brown Obsessive-Compulsive Scale (DY-BOCS): an instrument for assessing obsessive-compulsive symptom dimensions. Mol Psychiatry. 2006;11:495–504. doi: 10.1038/sj.mp.4001798. [DOI] [PubMed] [Google Scholar]
- 27.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Maldjian JA, Laurienti PJ, Burdette JB, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 29.Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayasaka S, Phan KL, Liberzon I, et al. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–87. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 31.Piras F, Piras F, Chiapponi C, et al. Widespread structural brain changes in OCD: a systematic review of voxel-based morphometry studies. Cortex. 2015;62C:89–108. doi: 10.1016/j.cortex.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Piras F, Piras F, Caltagirone C, et al. Brain circuitries of obsessive compulsive disorder: a systematic review and meta-analysis of diffusion tensor imaging studies. [Review] Neurosci Biobehav Rev. 2013;37:2856–77. doi: 10.1016/j.neubiorev.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–73. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Woods DW, Piacentini J, Himle MB, et al. Premonitory Urge for Tics Scale (PUTS): initial psychometric results and examination of the premonitory urge phenomenon in youths with tic disorders. J Dev Behav Pediatr. 2005;26:397–403. doi: 10.1097/00004703-200512000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Shavitt RG, Belotto C, Curi M, et al. Clinical features associated with treatment response in obsessive-compulsive disorder. Compr Psychiatry. 2006;47:276–81. doi: 10.1016/j.comppsych.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Koprivová J, Horácek J, Tintera J, et al. Medial frontal and dorsal cortical morphometric abnormalities are related to obsessive-compulsive disorder. Neurosci Lett. 2009;464:62–6. doi: 10.1016/j.neulet.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Shin YW, Yoo SY, Lee JK, et al. Cortical thinning in obsessive compulsive disorder. Hum Brain Mapp. 2007;28:1128–35. doi: 10.1002/hbm.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoo SY, Roh MS, Choi JS, et al. Voxel-based morphometry study of gray matter abnormalities in obsessive-compulsive disorder. J Korean Med Sci. 2008;23:24–30. doi: 10.3346/jkms.2008.23.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.May A. Experience-dependent structural plasticity in the adult human brain. Trends Cogn Sci. 2011;15:475–82. doi: 10.1016/j.tics.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Rossi S, Bartalini S, Ulivelli M, et al. Hypofunctioning of sensory gating mechanisms in patients with obsessive-compulsive disorder. Biol Psychiatry. 2005;57:16–20. doi: 10.1016/j.biopsych.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Russo M, Naro A, Mastroeni C, et al. Obsessive-compulsive disorder: a “sensory-motor” problem? Int J Psychophysiol. 2014;92:74–8. doi: 10.1016/j.ijpsycho.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Berman BD, Horovitz SG, Morel B, et al. Neural correlates of blink suppression and the buildup of a natural bodily urge. Neuroimage. 2012;59:1441–50. doi: 10.1016/j.neuroimage.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhtz-Buschbeck JP, Gilster R, van der Horst C, et al. Control of bladder sensations: an fMRI study of brain activity and effective connectivity. Neuroimage. 2009;47:18–27. doi: 10.1016/j.neuroimage.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Farrell MJ, Cole LJ, Chiapoco D, et al. Neural correlates coding stimulus level and perception of capsaicin-evoked urge-to-cough in humans. Neuroimage. 2012;61:1324–35. doi: 10.1016/j.neuroimage.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 45.Ryczko D, Dubuc R. The multifunctional mesencephalic locomotor region. [Review] Curr Pharm Des. 2013;19:4448–70. doi: 10.2174/1381612811319240011. [DOI] [PubMed] [Google Scholar]
- 46.Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. [Review] Psychopharmacology (Berl) 2001;156:216–24. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- 47.Ahmari SE, Risbrough VB, Geyer MA, et al. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology. 2012;37:1216–23. doi: 10.1038/npp.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantovani A, Rossi S, Bassi BD, et al. Modulation of motor cortex excitability in obsessive-compulsive disorder: an exploratory study on the relations of neurophysiology measures with clinical outcome. Psychiatry Res. 2013;210:1026–32. doi: 10.1016/j.psychres.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]


