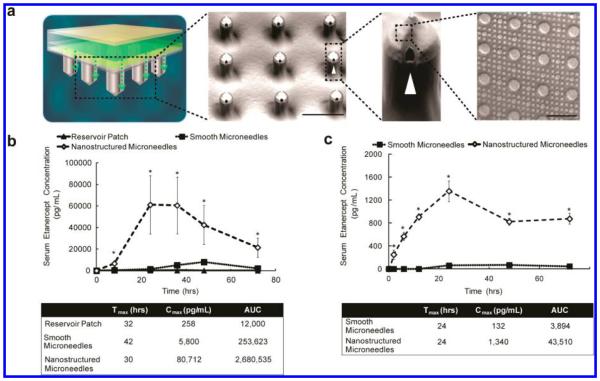
Figure 1.
Nanotopography significantly enhances transdermal delivery of etanercept in vivo. (a) Schematic representation of the transdermal delivery devices used in this study. Progressing from top to bottom: each device is made of an impermeable backing (tan), drug reservoir (green), rate-controlling membrane (yellow), and silicon microneedle array (MNA) (gray). Each 290 μm long, 100 μm wide microneedle has longitudinal perforations along the side, through which drug flows out. Drug flow from the reservoir down the grooves of the microneedles is indicated by a green dashed arrow. Perforations are denoted with a white arrowhead. “Smooth” microneedles were not coated with a film, while “nanostructured” microneedles were coated with a nanostructured film (scale bar represents 300 μm). Inset on furthest right depicts an SEM image of the nanostructures coated onto each microneedle (scale bar = 3 μm). (b) Nanostructured MNAs deliver significantly more etanercept transdermally than either drug reservoir patch alone or unstructured, smooth MNA controls in rats. After 72 h, the nanostructured MNA cumulatively delivered 10.6 times more etanercept (*p < 0.01) and achieved a maximal serum concentration (Cmax) 13.9 times higher (*p < 0.01) than the smooth microneedles (n = 4 animals for each category). (c) Nanostructured MNAs deliver significantly more etanercept transdermally than unstructured, smooth MNA controls in rabbits. After 72 h, the nanostructured MNA devices cumulatively delivered 35 times more etanercept (*p < 0.01) and achieved a Cmax 10.2 times higher than smooth MNA controls (*p < 0.01) (n = 4 animals for each category).