Figure 8.
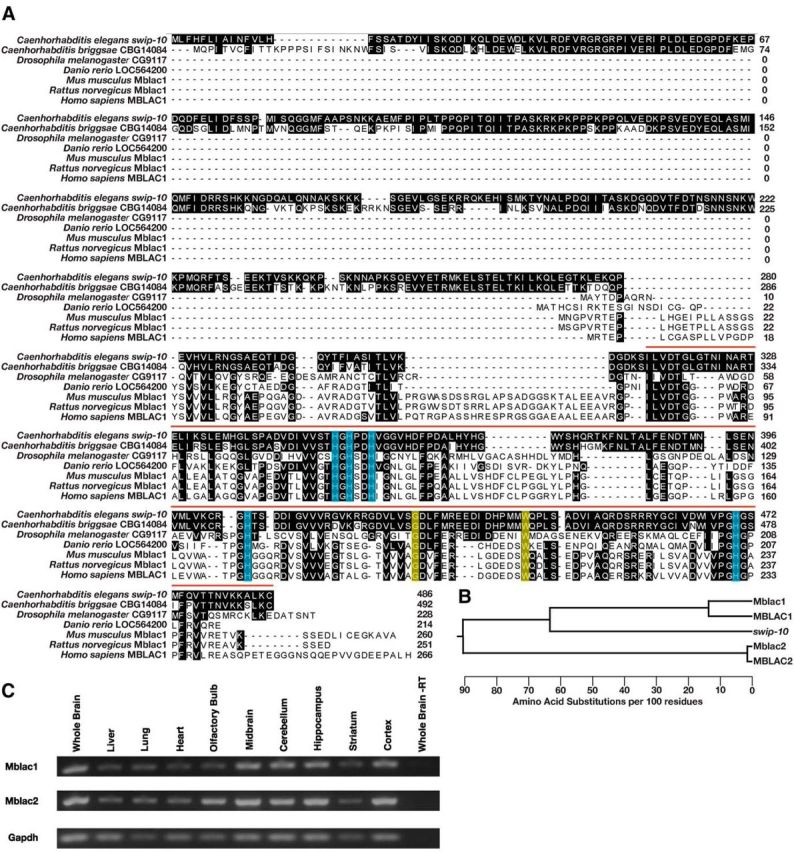
Phylogenetic conservation of swip-10 and CNS expression of the swip-10 mouse homolog Mblac1. A, The SWIP-10 protein exhibits significant conservation with its nematode homolog CBG14084 across phylogeny, concentrated at the protein's C-terminal MBD. The vertebrate homologs have much shorter protein sequences and only begin to align at 260 aa into the SWIP-10 protein sequence. Shaded residues indicate amino acids that match the SWIP-10 sequence. Red bar indicates the span of the MBD that begins with a canonical “ILVDTG” motif. Blue boxes indicate histidine residues that are predicted to be critical for metal binding, including a canonical HxHxDH motif that is typical of the entire metallo β-lactamase superfamily. Yellow boxes indicate amino acids that are altered in the strains isolated from the Swip-based mutagenesis screen. swip-10(vt29) harbors a SNP that results in the conversion of a conserved tryptophan at position 377 to a stop codon, resulting in a truncated MBD. The mutation in swip-10(vt33) converts a conserved glycine at position 362 to glutamic acid. Sequences were aligned using ClustalW (DNAStar). B, Dendrogram generated from multiple sequence alignment of swip-10 with Mblac1, MBLAC1, and another MBD containing protein in mouse and human named Mblac2 and MBLAC2, respectively. swip-10 exhibits greater identity with Mblac1 and MBLAC1. C, RT-PCR of Mblac1, Mblac2, and Gapdh from different areas of the mouse brain, heart, lung, and liver. We observed the presence of both RNAs in tissue harvested from all tissues assayed, but not in whole-brain lysates lacking reverse transcriptase.
